82890
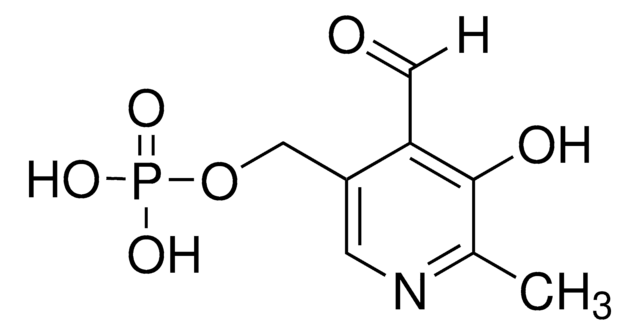
Pyridoxamine-5′-phosphate
≥98.0% (HPLC)
Synonym(s):
4-Aminomethyl-5-hydroxy-6-methyl-3-pyridylmethyl phosphate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H13N2O5P
CAS Number:
Molecular Weight:
248.17
Beilstein:
233653
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.79
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
form
powder
technique(s)
HPLC: suitable
color
faint beige to light beige
white to light yellow
SMILES string
Cc1ncc(COP(O)(O)=O)c(CN)c1O
InChI
1S/C8H13N2O5P/c1-5-8(11)7(2-9)6(3-10-5)4-15-16(12,13)14/h3,11H,2,4,9H2,1H3,(H2,12,13,14)
InChI key
ZMJGSOSNSPKHNH-UHFFFAOYSA-N
Related Categories
General description
Pyridoxamine is a derivative of vitamin B6. Pyridoxamine is a water-soluble vitamin. Vitamin B6 derivatives contain a core pyridine ring. Pyridoxaminecontains an amino methyl group at pyridine′s 4′ position.
Application
Pyridoxamine-5′-phosphate is suitable:
- in the enzymatic synthesis of cytidine diphosphate-4-keto-3, 6-dideoxyglucose
- as a substrate for pyridoxamine-5′-phosphate phosphatase activity assay
- as a substrate to characterize pyridoxine-5′-phosphate oxidase
- for the absorbance and fluorescence studies of properties of NaBH4-reduced glycogen phosphorylase b to probe the microenvironment of PMP residue
- as a substrate for characterization of pyridoxamine-phosphate transaminase(s) from various species and tissues
Biochem/physiol Actions
Pyridoxal 5′-phosphate can be converted to pyridoxamine-5′-phosphate in the presence of (S)-α-methylbenzylamine (MBA) as the amine donor by using pyruvate transaminase as a biocatalyst. Pyridoxine 5′-phosphate and pyridoxamine 5′-phosphate are oxidized to pyridoxal 5′-phosphate (PLP) using flavin mononucleotide (FMN) as the immediate electron acceptor and oxygen as the ultimate electron acceptor by the E. coli enzyme pyridoxine (pyridoxamine) 5′-phosphate oxidase.
pK of pyridoxamine-5′-phosphate (pyridoxamine 5-phosphate, PAMP) in the singlet excited state has been evaluated by absorption and fluorescence spectral studies.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I Miyahara et al.
Journal of biochemistry, 116(5), 1001-1012 (1994-11-01)
The three-dimensional structures of pyridoxamine 5'-phosphate-type aspartate aminotransferase from Escherichia coli and its complexes with maleate and glutarate have been determined by X-ray crystallography at 2.2, 2.1, and 2.7 A resolution, respectively. The enzyme is a dimeric form comprising two
Identification of a pyridoxine (pyridoxamine) 5'-phosphate oxidase from Arabidopsis thaliana.
Sang Y, Barbosa JM, Wu H, Locy RD, Singh NK.
Febs Letters, 2007, 344-348 (2007)
J D Choi et al.
The Journal of biological chemistry, 258(2), 840-845 (1983-01-25)
The kinetic properties of pyridoxamine (pyridoxine)-5'-phosphate oxidase have been studied using the physiological substrates pyridoxine 5'-phosphate (PNP) and pyridoxamine 5'-phosphate (PMP) at 25 degrees C and pH 8.0. Under steady-state conditions with different concentrations of PNP and O2, a series
Synthesis of pyridoxamine 5?-phosphate using an MBA: pyruvate transaminase as biocatalyst.
Schell U, et al.
Journal of Molecular Catalysis. B, Enzymatic, 59, 279-285 (2009)
Sonia Herrero et al.
Plant molecular biology, 76(1-2), 157-169 (2011-05-03)
Vitamin B6 (pyridoxal phosphate) is an essential cofactor in enzymatic reactions involved in numerous cellular processes and also plays a role in oxidative stress responses. In plants, the pathway for de novo synthesis of pyridoxal phosphate has been well characterized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service