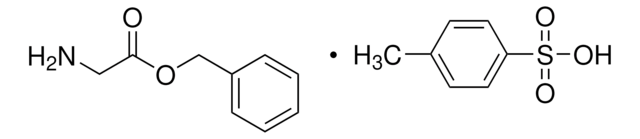
G3267
Glycine benzyl ester hydrochloride
≥98% (TLC)
Synonym(s):
Benzyl glycinate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H11NO2 · HCl
CAS Number:
Molecular Weight:
201.65
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
Glycine benzyl ester hydrochloride,
Assay
≥98% (TLC)
form
powder
color
white
storage temp.
−20°C
SMILES string
Cl.NCC(=O)OCc1ccccc1
InChI
1S/C9H11NO2.ClH/c10-6-9(11)12-7-8-4-2-1-3-5-8;/h1-5H,6-7,10H2;1H
InChI key
VLQHNAMRWPQWNK-UHFFFAOYSA-N
Application
Glycine benzyl ester is used as an organic reagent in condensing reactions.
Biochem/physiol Actions
The benzyl ester group of the amino acid monomers promote kinetically controlled synthesis (KCS) of polypeptides by increasing the substrate affinity and specificity of the enzyme. It is useful in the chemoenzymatic polymerization of glycine monomers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Martinez et al.
Carbohydrate research, 50(1), 15-22 (1976-08-01)
An O-glycodipeptide was synthesized by lengthening the peptide chain on the C-terminal side of a glycosylamino acid unit. N-(Benzyloxycarbonyl)-3-O-(2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl)-L-threonine o-nitrophenyl ester and pentachlorophenyl ester were condensed with glycine benzyl ester to give both the same glycodipeptide, [N-(benzyloxycarbonyl)-3-O-(2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl)-L-threonyl]glycine benzyl ester (9).
V Maurich et al.
Farmaco (Societa chimica italiana : 1989), 49(12), 805-808 (1994-12-01)
The synthesis of 2-(4-amino-5-chloro-2-methoxybenzamido)acetic acid 2, a metabolite of metoclopramide 1, has been accomplished through the coupling of 4-amino-5-chloro-2-methoxybenzoic acid 4 with glycine benzyl ester followed by a catalytic hydrogenation. Such a metabolite could not be detected directly in the
Jose Manuel Ageitos et al.
Biomacromolecules, 17(1), 314-323 (2015-12-02)
The chemoenzymatic polymerization of amino acid monomers by proteases involves a two-step reaction: the formation of a covalent acyl-intermediate complex between the protease and the carboxyl ester group of the monomer and the subsequent deacylation of the complex by aminolysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service