T3634
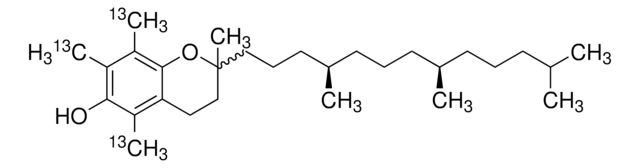
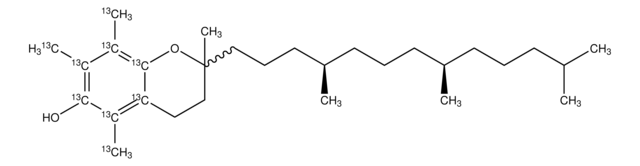
(+)-α-Tocopherol
from vegetable oil, Type V, ~1000 IU/g
Synonym(s):
2,5,7,8-Tetramethyl-2-(4′,8′,12′-trimethyltridecyl)-6-chromanol, 5,7,8-Trimethyltocol, D-α-Tocopherol, Vitamin E
About This Item
Recommended Products
biological source
vegetable oil
type
Type V
form
viscous liquid
specific activity
~1000 IU/g
color
clear yellow to red
refractive index
n20/D 1.505 (lit.)
bp
200-220 °C/0.1 mmHg (lit.)
density
0.95 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
CC(C)CCC[C@@H](C)CCC[C@@H](C)CCC[C@]1(C)CCc2c(C)c(O)c(C)c(C)c2O1
InChI
1S/C29H50O2/c1-20(2)12-9-13-21(3)14-10-15-22(4)16-11-18-29(8)19-17-26-25(7)27(30)23(5)24(6)28(26)31-29/h20-22,30H,9-19H2,1-8H3/t21-,22-,29-/m1/s1
InChI key
GVJHHUAWPYXKBD-IEOSBIPESA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as a food supplement for juvenile cobia fish
- to evaluate its protective effect on bisphenol A induced oxidative stress in rats
- to test its chemopreventive efficacy in estrogen-mediated mammary cancer rats
Biochem/physiol Actions
Packaging
Other Notes
Quality
Caution
Physical properties
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service