All Photos(3)
About This Item
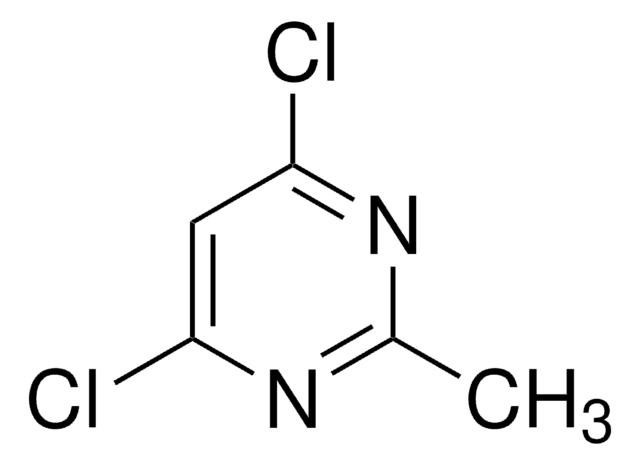
Empirical Formula (Hill Notation):
C5H4Cl2N2
CAS Number:
Molecular Weight:
163.00
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
219 °C (lit.)
mp
44-47 °C (lit.)
SMILES string
Cc1cc(Cl)nc(Cl)n1
InChI
1S/C5H4Cl2N2/c1-3-2-4(6)9-5(7)8-3/h2H,1H3
InChI key
BTLKROSJMNFSQZ-UHFFFAOYSA-N
General description
2,4-Dichloro-6-methylpyrimidine undergoes double cross-coupling reaction with 2-(tributylstannyl)pyridine, followed by aldol condensation to yield 4-arylvinyl-2,6-di(pyridin-2-yl)pyrimidines. It reacts with 1H,1H,2H,2H-perfluorodecanethiol during fluorous synthesis of disubstituted pyrimidines.
Application
2,4-Dichloro-6-methylpyrimidine was used in the synthesis of (2-chloro-6-methyl-pyrimidin-4-yl)-(2,3-dihydro-benzothiazol-6-yl)-amine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Caroline Hadad et al.
The Journal of organic chemistry, 76(10), 3837-3845 (2011-04-06)
A series of 4-arylvinyl-2,6-di(pyridin-2-yl)pyrimidines have been efficiently prepared by a double cross-coupling reaction between 2,4-dichloro-6-methylpyrimidine and 2-(tributylstannyl)pyridine, followed by aldol condensation with the appropriate aromatic aldehyde substituted with electron-donating, electron-withdrawing, dendritic, or water-soluble groups. The effect of different protic and
Eliud Hernández et al.
Puerto Rico health sciences journal, 29(4), 348-356 (2011-01-26)
Rho family GTPases are molecular switches that control signaling pathways regulating a myriad of cellular functions. Rac1, a Rho family member, plays a critical role in several aspects of tumorigenesis, cancer progression, invasion, and metastasis. Rac proteins are not mutated
Wei Zhang
Organic letters, 5(7), 1011-1013 (2003-03-28)
[reaction: see text] The fluorous synthesis of disubstituted pyrimidines is carried out by attaching 2,4-dichloro-6-methylpyrimidine with 1H,1H,2H,2H-perfluorodecanethiol. The tagged substrate is substituted with 3-(trifluoromethyl)pyrazole followed by thioether oxidation and tag displacement with amines or thiols. The fluorous chain serves as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)