15035
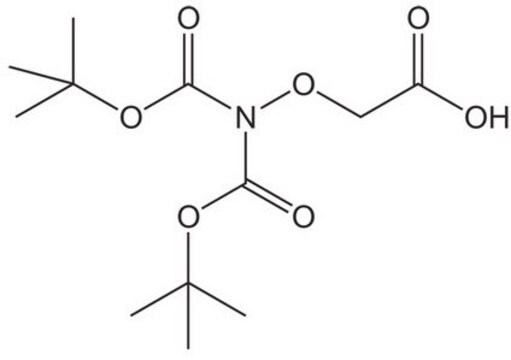
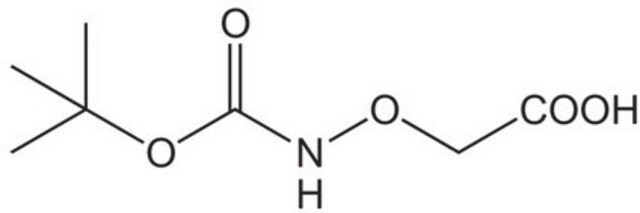
(Boc-aminooxy)acetic acid
≥98.0% (T)
Synonym(s):
N-Boc-(carboxymethoxy)amine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CO2CNHOCH2CO2H
CAS Number:
Molecular Weight:
191.18
Beilstein:
6137646
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (T)
form
powder
mp
~115 °C (dec.)
SMILES string
CC(C)(C)OC(=O)NOCC(O)=O
InChI
1S/C7H13NO5/c1-7(2,3)13-6(11)8-12-4-5(9)10/h4H2,1-3H3,(H,8,11)(H,9,10)
InChI key
QBXODCKYUZNZCY-UHFFFAOYSA-N
Application
(Boc-aminooxy)acetic acid was used in the preparation of Boc-aminooxy tetra(ethylene glycol).
Other Notes
Employed to introduce a hydroxylamine moiety into peptides; reaction with an aldehyde to an oxime.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mariarosaria De Simone et al.
Contrast media & molecular imaging, 11(6), 561-571 (2017-01-05)
Superparamagnetic iron oxide nanoparticles (SPIONs) have received increasing interest as contrast media in biomedical imaging and innovative therapeutic tools, in particular for loco-regional ablative treatments and drug delivery. The future of therapeutic applications would strongly benefit from improving the capability
Site-specific protein immobilization through N-terminal oxime linkages.
Journal of Materials Chemistry, 17(19), 2021-2027 (2007)
O. Nyanguile et al.
Lett. Pept. Sci., 1, 9-9 (1994)
J. Shao et al.
Journal of the American Chemical Society, 117, 3893-3893 (1995)
Rory K Morgan et al.
ACS chemical biology, 10(8), 1778-1784 (2015-05-16)
ADP-ribosylation is essential for cell function, yet there is a dearth of methods for detecting this post-translational modification in cells. Here, we describe a clickable aminooxy alkyne (AO-alkyne) probe that can detect cellular ADP-ribosylation on acidic amino acids following Cu-catalyzed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service