All Photos(1)
About This Item
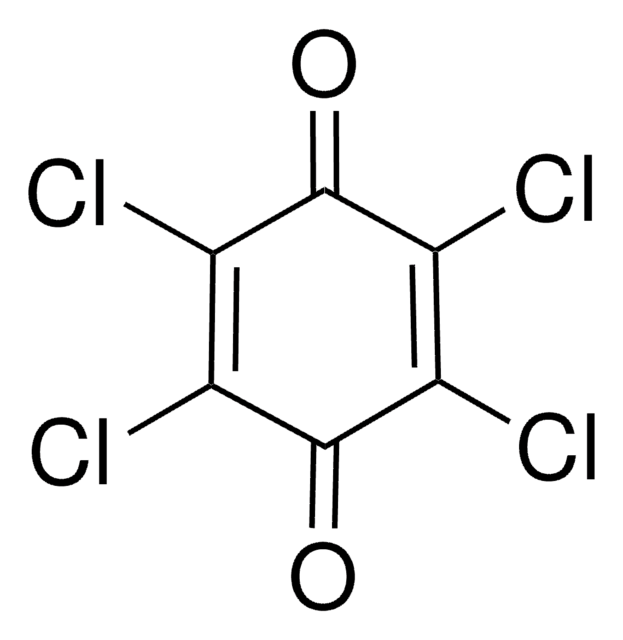
Empirical Formula (Hill Notation):
C6H3ClO2
CAS Number:
Molecular Weight:
142.54
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
SMILES string
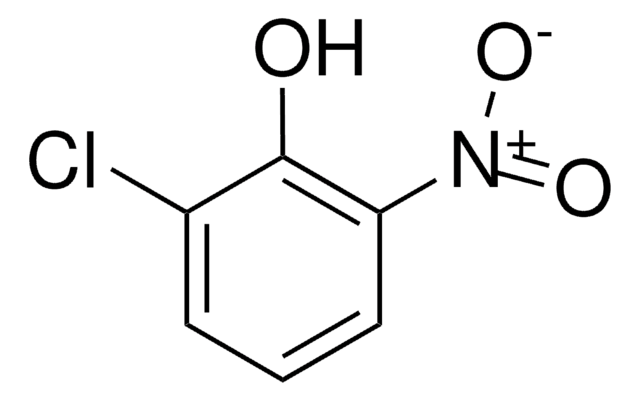
ClC1=CC(=O)C=CC1=O
InChI
1S/C6H3ClO2/c7-5-3-4(8)1-2-6(5)9/h1-3H
InChI key
WOGWYSWDBYCVDY-UHFFFAOYSA-N
Related Categories
General description
2-Chloro-1,4-benzoquinone is a quinone derivative. It is one of the intermediate formed during the degradation of 3,4-dichloroaniline in a dielectric barrier discharge plasma reactor. It is formed during lignin peroxidase catalyzed oxidation of 2-chloro-1,4-dimethoxybenzene. 2-Chloro-1,4-benzoquinone on dechlorination yields 1,2,4-trihydroxybenzene.
Application
2-Chloro-1,4-benzoquinone may be used in the preparation of chloro derivatives of prenylnaphthohydroquinone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An ecologically effective water treatment technique using electrochemically generated hydroxyl radicals for in situ destruction of organic pollutants: Application to herbicide 2, 4-D.
Oturan MA.
J. Appl. Electrochem., 30(4), 475-482 (2000)
R Grey et al.
Journal of basic microbiology, 38(5-6), 371-382 (1999-01-01)
The white-rot fungus Trametes versicolor was used to study the influence of extracellular laccase activity on the degradation of 2-chlorophenol (2-CP) and the formation of metabolites under conditions, characterized by the absence of other phenol-oxidizing enzymes. 2-CP enhanced the production
Emily N Vebrosky et al.
Journal of agricultural and food chemistry, 67(27), 7609-7615 (2019-07-02)
Shallow water systems are uniquely susceptible to environmental processes such as photolysis and hydrolysis that can influence the dissipation of pesticides into sediments. The fungicide dicloran has previously been shown to undergo photolysis and is reported to dissipate in soils
Aurora Molinari et al.
Bioorganic & medicinal chemistry, 13(11), 3841-3846 (2005-06-21)
From the Diels-Alder adduct between alpha-myrcene and 2-chloro-1,4-benzoquinone, a family of chloro derivatives of prenylnaphthohydroquinone have been synthesised and evaluated for their cytotoxicity against 14 neoplastic cell lines.
P J Teunissen et al.
Archives of biochemistry and biophysics, 360(2), 233-238 (1998-12-16)
2-Chloro-1,4-dimethoxybenzene (2Cl-1,4-DMB) oxidation by lignin peroxidase (LiP) proceeds via the formation of the 2Cl-1,4-DMB cation radical as indicated by ESR and UV/vis spectroscopy. The products of the LiP-catalyzed oxidation of 2Cl-1,4-DMB were identified as 2-chloro-1,4-benzoquinone and the dimers dichlorotetramethoxybiphenyl and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service