412929
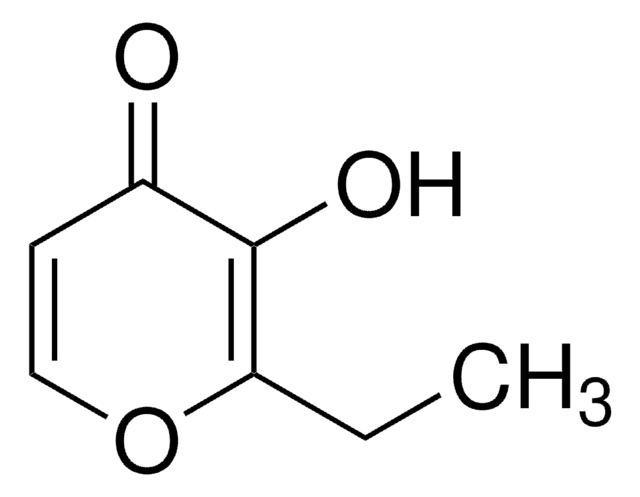
2-Ethyl-3-hydroxy-4H-pyran-4-one
99%
Synonym(s):
Ethyl maltol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H8O3
CAS Number:
Molecular Weight:
140.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
85-95 °C (lit.)
SMILES string
CCC1=C(O)C(=O)C=CO1
InChI
1S/C7H8O3/c1-2-6-7(9)5(8)3-4-10-6/h3-4,9H,2H2,1H3
InChI key
YIKYNHJUKRTCJL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Synthesis and Anti-Oomycete Mechanism: Research focused on the synthesis of sulfonate derivatives of Ethyl Maltol, incorporating 2-Ethyl-3-hydroxy-4H-pyran-4-one, and their application in combating oomycete pathogens, providing insights into the preliminary mechanisms that enhance agricultural productivity and disease management (Xing et al., 2022).
- Redox Activity in Co(III) Complexes: A study examined the effects of replacing traditional tripodal donors with two 2N chelators in Co(III) complexes containing maltolato (a derivative of 2-Ethyl-3-hydroxy-4H-pyran-4-one) on their redox behavior and cytotoxic activity, which is crucial for the development of novel therapeutic agents (Nagy et al., 2021).
- Characterization of Wine Aroma Compounds: The role of 2-Ethyl-3-hydroxy-4H-pyran-4-one in the characterization of odor-active compounds in various cherry wines was explored using gas chromatography-mass spectrometry and olfactometry, highlighting its significance in enhancing sensory attributes and wine quality (Niu et al., 2011).
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mutlu Dilsiz Aytemir et al.
Archiv der Pharmazie, 337(5), 281-288 (2004-04-20)
In this study, thirteen 3-hydroxy-6-methyl-2-substituted 4H-pyran-4-one derivatives were synthesized for the evaluation of their potential anticonvulsant activity. Mannich bases were prepared by the reaction of substituted piperazine derivatives with allomaltol and formaline. The structures of the synthesized compounds were confirmed
Carlyle Ribeiro Lima et al.
Molecules (Basel, Switzerland), 19(7), 9591-9605 (2014-07-09)
Tyrosinase is a key enzyme in melanin synthesis and widely distributed in plants and animals tissues. In mammals, this enzyme is related to pigment production, involved in wound healing, primary immune response and it can also contribute to catecholamines synthesis
Yunwei Niu et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 879(23), 2287-2293 (2011-07-06)
To characterize the aroma of cherry wine, five samples were analyzed by quantitative descriptive sensory analysis, gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O). The aroma of cherry wines was described by 6 sensory terms as fruity, sour, woody, fermentation
Volatile and semi-volatile components of oak wood chips analysed by accelerated solvent extraction (ASE) coupled to gas chromatography-mass spectrometry (GC-MS).
Vichi S, et al.
Food Chemistry, 102(4), 1260-1269 (2007)
R D Abeysinghe et al.
European journal of nuclear medicine, 21(10), 1141-1147 (1994-10-01)
In order to identify new compounds which label platelets without affecting their function, three classes of metal chelating agents have been compared with oxine for their efficiency of indium-113m platelet labelling and for their short- and long-term effects on platelet
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service