511420
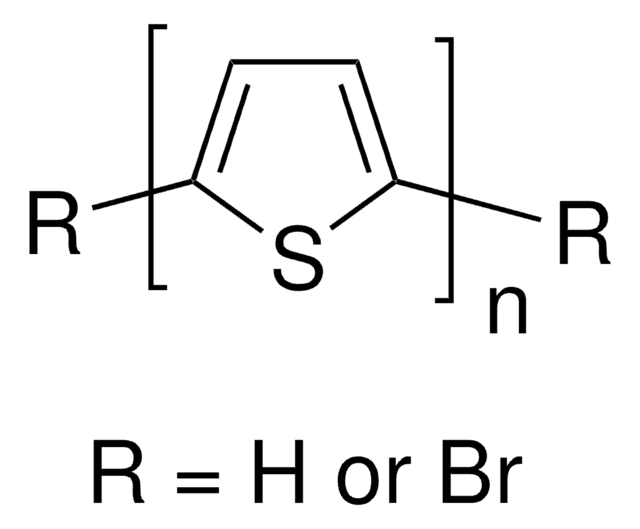
Poly(3-butylthiophene-2,5-diyl)
regiorandom
Synonym(s):
P3BT
About This Item
Recommended Products
color
red
solubility
chloroform, methylene chloride, toluene, and THF: soluble
fluorescence
λex 419 nm; λem 550 nm in chloroform
InChI
1S/C10H16S/c1-4-5-6-10-7-8(2)11-9(10)3/h7H,4-6H2,1-3H3
InChI key
DUOSBQJOYVIVOR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Packaging
Citation
Legal Information
Rieke is a registered trademark of Rieke Metals, Inc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The application of conducting polymers at the interface with biology is an exciting new trend in organic electronics research.
Novel Graphene‑Based Nanostructures Production, Functionalization, and Engineering
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Progress in solution-processed functional materials leads to thin-film optoelectronic devices for industrial and consumer electronics.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service