678740
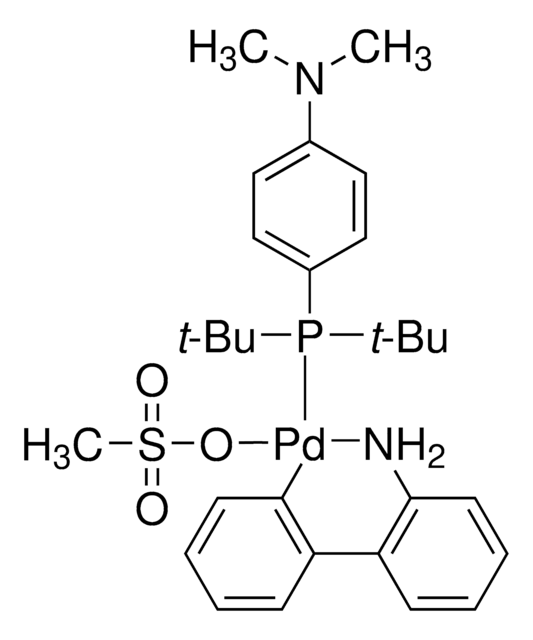
Bis(di-tert-butyl(4-dimethylaminophenyl)phosphine)dichloropalladium(II)
Synonym(s):
(A-taPhos)2PdCl2, Pd(amphos)Cl2
About This Item
Recommended Products
form
solid
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
functional group
phosphine
SMILES string
Cl[Pd]Cl.CN(c1ccc(P(C(C)(C)C)C(C)(C)C)cc1)C.CN(c2ccc(P(C(C)(C)C)C(C)(C)C)cc2)C
InChI
1S/2C16H28NP.2ClH.Pd/c2*1-15(2,3)18(16(4,5)6)14-11-9-13(10-12-14)17(7)8;;;/h2*9-12H,1-8H3;2*1H;/q;;;;+2/p-2
InChI key
DWOZNANUEDYIOF-UHFFFAOYSA-L
General description
For small scale and high throughput uses, product is also available as ChemBeads (927791)
Application
- In the enantioselective construction of indole-fused bicyclo[3.2.1]-octanes via an aminopalladition-triggered Heck-type reaction.
- In the synthesis of phenanthridine derivatives from ortho bromo N-tosylhydrazones and 2-aminophenylboronic ester via Suzuki cross-coupling reaction followed by intramolecular condensation reaction.
- In the synthesis of indenones by Pd-catalyzed annulation of an ortho-halobenzyl alcohol with internal alkynes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Ligand used to prepare a palladium dichloride catalyst on treatment with PdCl2(COD). The catalyst effectively cross-couples aryl boronic acids with heteroaryl chlorides.
A variety of palladium-catalyzed cross-coupling reactions can be run under room temperature conditions in water with TPGS- 750-M, using a variety of commercially available palladium complexes and ligands.
Protocols
TPGS-750-M surfactant enables various reactions in water at room temperature, enhancing efficiency and versatility in synthesis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
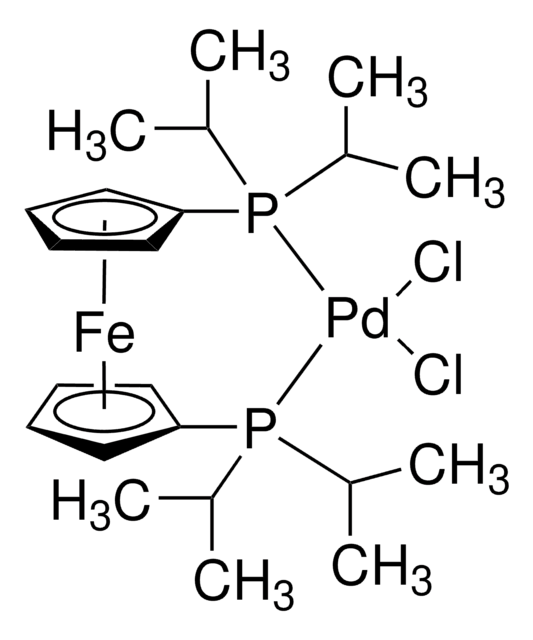
Contact Technical Service![[1,1′-Bis(di-tert-butylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)

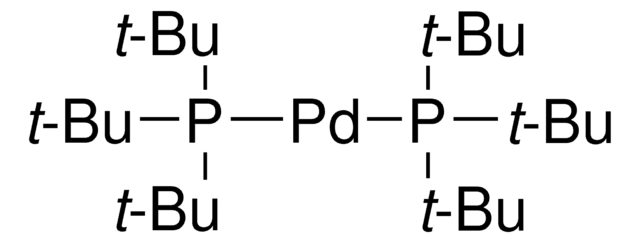

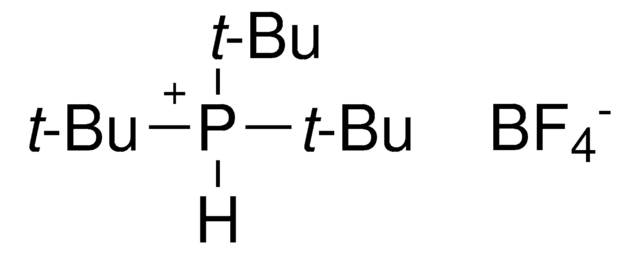


![[1,1′-Bis(di-cyclohexylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/136/854/a3142b2e-900c-47e5-8100-e48add9f4db6/640/a3142b2e-900c-47e5-8100-e48add9f4db6.png)