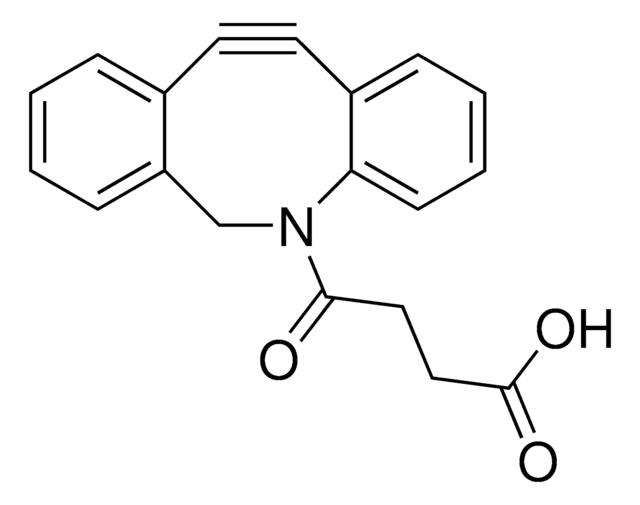
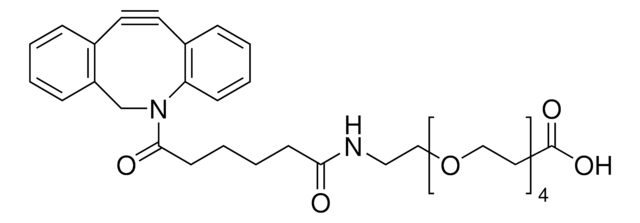
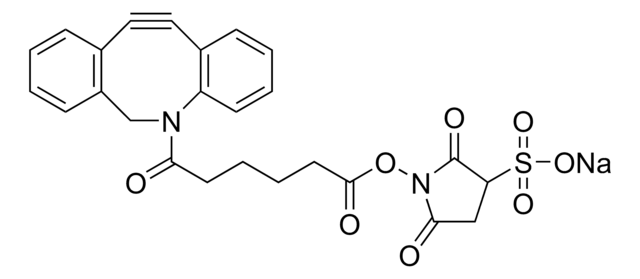
742678
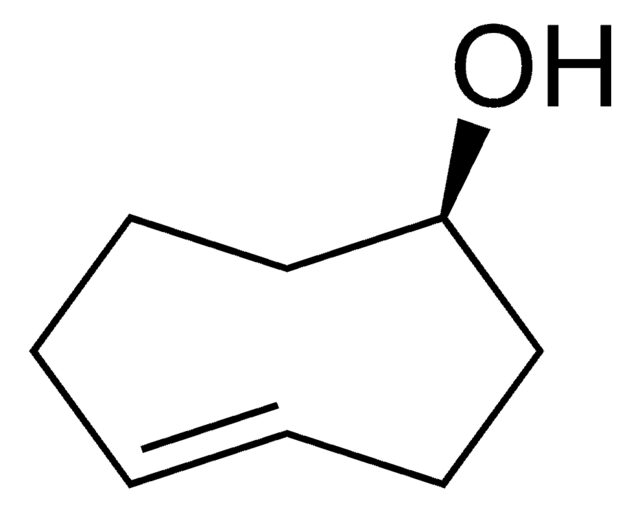
(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethanol
for Copper-free Click Chemistry
Synonym(s):
(1α,8α,9β)-Bicyclo[6.1.0]non-4-yne-9-methanol, endo-9-Hydroxymethylbicyclo[6.1.0]non-4-yne, BCN-OH
About This Item
Recommended Products
form
powder
composition
carbon content, 79.96%
hydrogen content, 9.39%
reaction suitability
reaction type: click chemistry
reagent type: linker
functional group
hydroxyl
storage temp.
−20°C
SMILES string
[H][C@@]12[C@@]([C@H]2CO)([H])CCC#CCC1
InChI
1S/C10H14O/c11-7-10-8-5-3-1-2-4-6-9(8)10/h8-11H,3-7H2/t8-,9+,10-
InChI key
NSVXZMGWYBICRW-ILWJIGKKSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Packaging
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service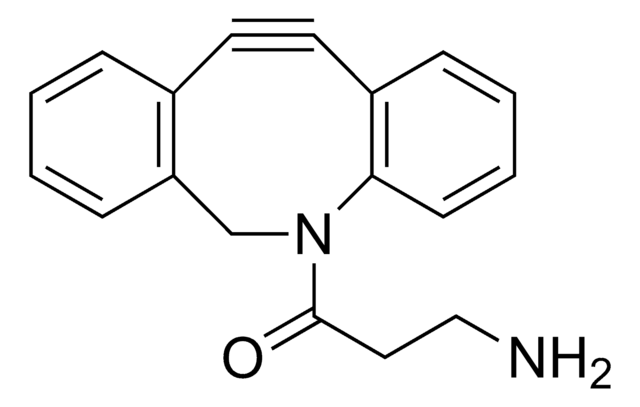
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)
![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)