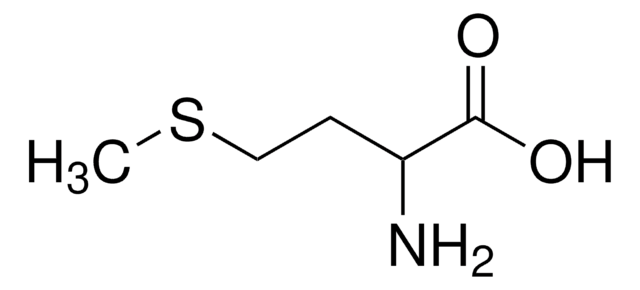
W326305
L-Cysteine
≥97%, FG
Synonym(s):
(R)-2-Amino-3-mercaptopropionic acid
About This Item
Recommended Products
grade
FG
Halal
reg. compliance
EU Regulation 1334/2008 & 872/2012
FDA 21 CFR 172.320
FDA 21 CFR 184.1271
Assay
≥97%
optical activity
[α]26/D +8.0 to +9.5°, c = 2 in 5 M HCl
mp
240 °C (dec.) (lit.)
solubility
H2O: 25 mg/mL
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
Organoleptic
sulfurous
SMILES string
N[C@@H](CS)C(O)=O
InChI
1S/C3H7NO2S/c4-2(1-7)3(5)6/h2,7H,1,4H2,(H,5,6)/t2-/m0/s1
InChI key
XUJNEKJLAYXESH-REOHCLBHSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Biochem/physiol Actions
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service