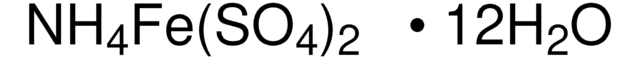1.09079
Ammonium thiocyanate solution
c(NH₄SCN) = 0.1 mol/l (0.1 N), reag. Ph. Eur., reag. USP, ready-to-use for titration, Titriplex®
Synonym(s):
Ammoniumrhodanid solution
About This Item
Recommended Products
product name
Ammonium thiocyanate solution, c(NH4SCN) = 0.1 mol/l (0.1 N), Titripur®, reag. Ph. Eur., reag. USP
Agency
reag. Ph. Eur.
reag. USP
Quality Level
product line
Titripur®
form
liquid
quality
Analyzed in our ISO 17025 accredited QC lab
reaction suitability
reaction type: Precipitation reactions
concentration
0.1 M
technique(s)
titration: suitable
pH
4.5 (20 °C in H2O)
density
1.00 g/cm3 at 20 °C
storage temp.
15-25°C
Application
- Thiocyanate-Treated Perovskite-Nanocrystal-Based Light-Emitting Diodes with Insight in Efficiency Roll-Off.: This study explores the use of thiocyanate treatments on perovskite nanocrystals to improve the performance and efficiency of light-emitting diodes (LEDs). The research highlights the potential of ammonium thiocyanate solution in enhancing the optoelectronic properties of perovskite-based LEDs, offering insights into efficiency roll-off mechanisms (Chen et al., 2020).
- Specific determination of ferbam residues in fog-water.: This paper discusses a method for detecting ferbam residues in fog-water using ammonium thiocyanate solution. The approach is significant for environmental analytical chemistry, providing a specific and sensitive detection method for fungicide residues in environmental samples (Agarwal et al., 2003).
- Flow-injection simultaneous determination of iron(III) and copper(II) and of iron(III) and palladium(II) based on photochemical reactions of thiocyanato-complexes.: This research presents a flow-injection analysis technique for the simultaneous determination of iron and copper or iron and palladium using photochemical reactions involving ammonium thiocyanate solution. It demonstrates the solution′s utility in multi-element analysis in complex matrices (Oguma and Yoshioka, 2002).
- Colorimetric determination of zirconium with 1-(2-pyridylazo)-2-naphthol.: This study involves the use of ammonium thiocyanate solution in the colorimetric determination of zirconium. The method offers a simple and effective way to measure zirconium concentrations, relevant in both industrial and environmental analytical applications (Ross et al., 1969).
- The thiocyanate titration of mercury and the standardization of ammonium thiocyanate solution.: This classic paper details the titration of mercury using ammonium thiocyanate solution, providing standardized methods for analytical applications. It highlights the fundamental role of thiocyanate solutions in titrimetric analyses (Cumming and Spice, 1952).
Features and Benefits
This volumetric solution is analyzed by our calibration laboratory D-K-15185-01-00 which is accredited according to DIN EN ISO/IEC 17025 for analysis of amount-of-substance concentrations in volumetric solutions by DAkkS (Deutsche Akkreditierungsstelle - German National Accreditation Body). The accreditation certificate can be found at www.sigmaaldrich.com/ISO17025.
Packaging
Analysis Note
Amount-of-substance concentration 0.0995 - 0.1005 mol/L
Measurement uncertainty ± 0.0003 mol/L
Traceability NIST SRM
The concentration is determined by volumetric titration and refers to 20°C.
The amount-of-substance concentration of this volumetric solution is determined with standardized silver nitrate solution (article number 1.09081). The silver nitrate solution is standardized and traceable to a primary standard reference material (SRM) from the National Institute of Standards and Technology, Gaithersburg, USA (NIST SRM 999 potassium chloride) by means of volumetric standard sodium chloride (article number 1.02406), certified reference material according to ISO 17034, analyzed by our accredited calibration laboratory of Merck KGaA, Darmstadt, Germany according to DIN EN ISO/IEC 17025. The uncertainty is expressed as expanded measurement uncertainty with a coverage factor k=2 covering a confidence level of 95%.
Note: The titer is a correction factor to correct for variations of the volumetric solution, the titration equipment, the temperature and other laboratory conditions. For correct titration results it is recommended to determine a titer with the laboratory specific equipment and under laboratory specific conditions directly after opening a new bottle and at regular time intervals.
Legal Information
related product
Storage Class Code
12 - Non Combustible Liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
SmartChemicals enable wireless CoA data transfer, reducing human error in titration experiments.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service