1.46463
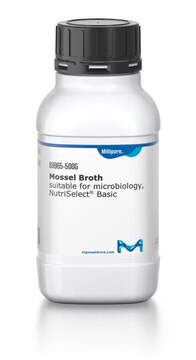
Mossel Broth
ready-to-use, bottle volume 100 mL , filling volume
Synonym(s):
Buffered Glucose-Brilliant Green Bile Broth, EE Broth, Enterobacteria Enrichment Broth Mossel
About This Item
Recommended Products
Agency
EP 2.6.1
USP
Quality Level
description
For selective enrichment Enterobacteriaceae; Suitable for sterility testing
sterility
sterile; autoclaved
form
liquid
shelf life
8 mo.
feature
closure type screw cap with septum
ready-to-use
packaging
bottle of
single packed of (100 ml in 125 ml bottle with blue screw cap and 3 loci (10 bottles per box))
technique(s)
direct inoculation: suitable
sterile filtration: suitable
bottle capacity
125 mL
bottle volume
100 mL , filling volume
pH
7.2
application(s)
bioburden testing
cosmetics
food and beverages
pharmaceutical
storage temp.
15-25°C
suitability
Enterobacter spp.
nonselective for
General description
Application
Features and Benefits
- Ready-to-use media
- Bottles with screw caps
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Learn more about our culture media portfolio for microbial examination of non-sterile products, fully compliant with the harmonized Pharmacopoeia.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service