MAB8261
Anti-Influenza A Antibody, H1N1, clone 9B3.2
clone 9B3.2, Chemicon®, from mouse
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
clone
9B3.2, monoclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
immunofluorescence: suitable
isotype
IgG2a
shipped in
wet ice
Specificity
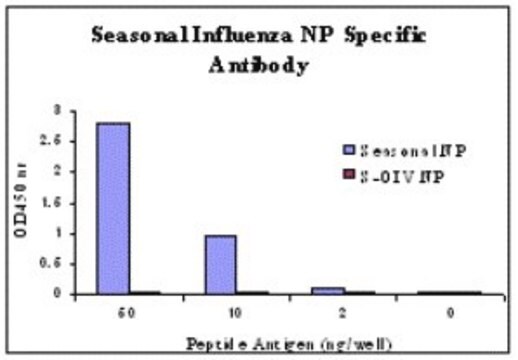
Influenza A H1N1 antigen. No reactivity shown to Influenza B strains.
SPECIES REACTIVITY:
Reacts strongly with the following H1N1 strain: Beijing. A/ Texas /36/91, A/Berkeley/1/98, A/HongKong/503/97,A/Nanchang /16A/98,A/PR8/34 and New Caledoniastrain.
SPECIES REACTIVITY:
Reacts strongly with the following H1N1 strain: Beijing. A/ Texas /36/91, A/Berkeley/1/98, A/HongKong/503/97,A/Nanchang /16A/98,A/PR8/34 and New Caledoniastrain.
Immunogen
Epitope: H1N1
Influenza blend
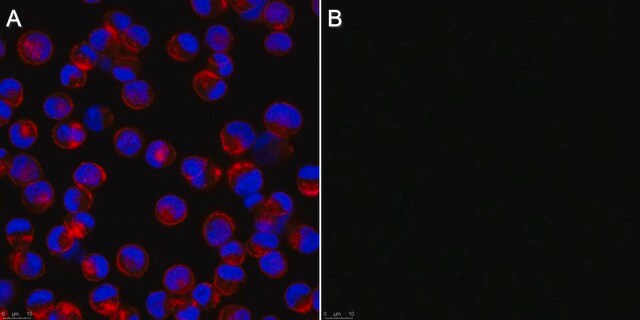
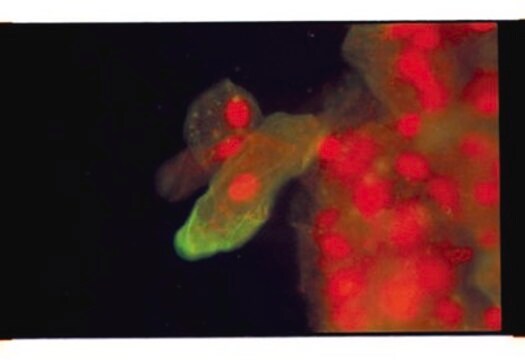
Application
IF
Optimal dilutions must be determined by end user
Optimal dilutions must be determined by end user
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
This Anti-Influenza A Antibody, H1N1, clone 9B3.2 is validated for use in IF for the detection of Influenza A.
Physical form
Format: Purified
Purified immunoglobulin. Liquid in 0.02 M Phosphate Buffer + 0.25 M Sodium Chloride + 0.1% Sodium Azide, pH = 7.6
Storage and Stability
Store at 2° to 8°C for up to 12 months from date of receipt.
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Claudia Bank et al.
Evolution; international journal of organic evolution, 70(11), 2470-2484 (2016-11-01)
The rapid evolution of drug resistance remains a critical public health concern. The treatment of influenza A virus (IAV) has proven particularly challenging, due to the ability of the virus to develop resistance against current antivirals and vaccines. Here, we
Evolution of the influenza A virus genome during development of oseltamivir resistance in vitro.
Renzette, N; Caffrey, DR; Zeldovich, KB; Liu, P; Gallagher, GR; Aiello, D; Porter et al.
Journal of virology null
Li Jiang et al.
Journal of virology, 96(6), e0198221-e0198221 (2022-01-20)
Many oseltamivir resistance mutations exhibit fitness defects in the absence of drug pressure that hinders their propagation in hosts. Secondary permissive mutations can rescue fitness defects and facilitate the segregation of resistance mutations in viral populations. Previous studies have identified
Kristina L Prachanronarong et al.
Journal of virology, 93(2) (2018-11-02)
Influenza A virus (IAV), a major cause of human morbidity and mortality, continuously evolves in response to selective pressures. Stem-directed, broadly neutralizing antibodies (sBnAbs) targeting the influenza virus hemagglutinin (HA) are a promising therapeutic strategy, but neutralization escape mutants can
Sialylneolacto-N-tetraose c (LSTc)-bearing liposomal decoys capture influenza A virus.
Hendricks, GL; Weirich, KL; Viswanathan, K; Li, J; Shriver, ZH; Ashour, J; Ploegh et al.
The Journal of Biological Chemistry null
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service