07964
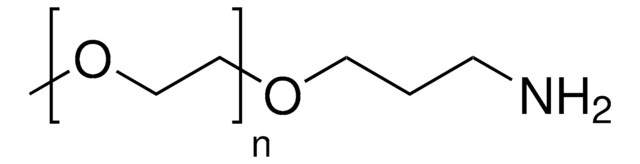
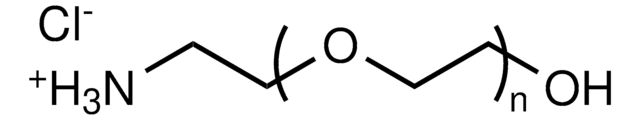
Methoxypolyethylene glycol amine
750
Synonym(s):
mPEG-NH2, O-(2-Aminoethyl)-O′-methylpolyethylene glycol, Aminopolyethylene glycol monomethyl ether, Methoxypolyoxyethylene amine
About This Item
Recommended Products
Quality Level
form
solid
color
white to yellow
InChI
1S/C3H9NO/c1-5-3-2-4/h2-4H2,1H3
InChI key
ASUDFOJKTJLAIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Form dendritic micelles to enhance the solubility of hydrophobic compounds.
- Conjugate with an anticancer drug methotrexate (MTX) to form MTX-PEG with potential application as drug carriers.
- Couple with polyethylenimine (PEI) to form graft copolymers which can be used as nonviral gene vectors.
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Biofouling control essential for device performance and safety; minimize accumulation of biomolecules and bioorganisms.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service