11630
2,2′-Azobis(2-methylpropionitrile)
purum, ≥98.0% (GC)
Synonym(s):
α,α′-Azoisobutyronitrile, AIBN, Azobisisobutyronitrile, Free radical initiator
About This Item
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (GC)
form
solid
impurities
≤2% water
ign. residue
≤0.1%
mp
102-104 °C (dec.) (lit.)
storage temp.
2-8°C
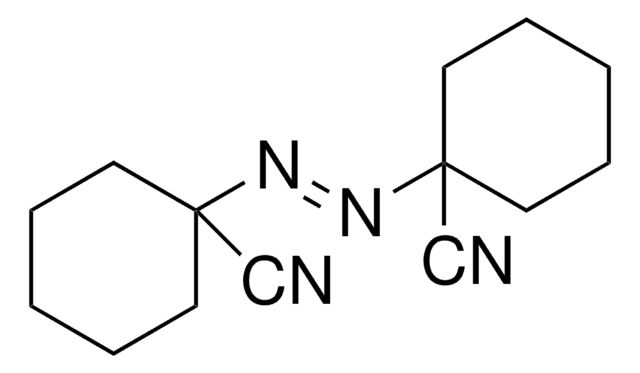
SMILES string
CC(C)(\N=N\C(C)(C)C#N)C#N
InChI
1S/C8H12N4/c1-7(2,5-9)11-12-8(3,4)6-10/h1-4H3/b12-11+
InChI key
OZAIFHULBGXAKX-VAWYXSNFSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Polymer Chemistry: Analysis of polymer synthesis using AIBN under various conditions, with emphasis on thermal initiation properties (Suriyanarayanan & Nicholls, 2019).
- Influence of cyclodextrin on the UCST-and LCST-behavior of poly (2-methacrylamido-caprolactam)-co-(N, N-dimethylacrylamide): Explores the role of AIBN in copolymerization processes influencing polymer properties relevant to material science applications (Burkhart & Ritter, 2014).
- Preparation and characterization of polyaniline microcapsule loaded with 2, 2′‐azobis (2‐methylpropionitrile) initiator and its controlled release: Demonstrates innovative applications of AIBN in microencapsulation techniques, targeting enhanced performance in adhesive industries (Zhang et al., 2022).
Storage and Stability
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Self-react. C
Supplementary Hazards
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 2
Flash Point(F)
122.0 °F
Flash Point(C)
50 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service