63978
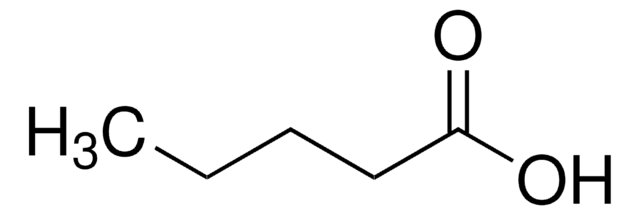
(RS)-(Methylenecyclopropyl)acetic acid
analytical standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8O2
CAS Number:
Molecular Weight:
112.13
Beilstein:
1927126
MDL number:
UNSPSC Code:
85151701
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
Assay
≥95.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
food and beverages
format
neat
storage temp.
−20°C
SMILES string
OC(=O)CC1CC1=C
InChI
1S/C6H8O2/c1-4-2-5(4)3-6(7)8/h5H,1-3H2,(H,7,8)
InChI key
QJBXAEKEXKLLLZ-UHFFFAOYSA-N
Application
(RS)-(Methylenecyclopropyl)acetic acid (MCPA), an inhibitor of multiple acyl-CoA dehydrogenase enzymes, is derived from hypoglycin A metabolism. MCPA forms non-metabolizable carnitine and coenzyme A (CoA) esters thereby depressing tissue levels of these cofactors and making them less available for other biochemical reactions. (RS)-(Methylenecyclopropyl)acetic acid may be used as a reference material during the analysis of MCPA.
Hypoglycin A is metabolized by means of transamination and oxidative decarboxylation to methylene cyclopropyl acetic acid (MCPA). MCPA forms nonmetabolizable carnitine and coenzyme A (CoA) esters, thereby depressing tissue levels of these cofactors and making them less available for other biochemical reactions.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Recommended products
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Y K Lieu et al.
The American journal of physiology, 272(3 Pt 1), E359-E366 (1997-03-01)
To examine the changes in coenzyme A profile and the possible corrective effects of carnitine supplementation in the genetic disorders of mitochondrial beta-oxidation, we carried out experiments using an inhibitor of multiple acyl-CoA dehydrogenase enzymes, methylenecyclopropaneacetic acid (MCPA), in rat
Methylenecyclopropaneacetic acid, a metabolite of hypoglycin.
C Von Holt
Biochimica et biophysica acta, 125(1), 1-10 (1966-08-03)
K Y Tserng et al.
Biochemistry, 30(44), 10755-10760 (1991-11-05)
To study the structure-activity relationship between pentanoic acid analogues and the inhibition of fatty acid oxidation, a number of 4-pentenoic and methylenecyclopropaneacetic acid derivatives were prepared. All compounds inhibited palmitoylcarnitine oxidation in rat liver mitochondria, with 50% inhibition occurring at
The antagonism of the toxicity of hypoglycin by glycine.
S S Al-Bassam et al.
Biochemical pharmacology, 30(20), 2817-2824 (1981-10-01)
David Grünig et al.
Biochemical pharmacology, 177, 113860-113860 (2020-03-14)
Treatment with valproate is associated with hepatic steatosis, but the mechanisms are not fully elucidated in human cell systems. We therefore investigated the effects of valproate on fatty acid and triglyceride metabolism in HepaRG cells, a human hepatoma cell line.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service