About This Item
Recommended Products
biological source
synthetic
Quality Level
sterility
non-sterile
Assay
≥95% (HPLC)
form
powder
technique(s)
cell based assay: suitable
solubility
1% acetic acid: 1.00-1.04 mg/mL, clear, colorless
water: 1.00-1.04 mg/mL, clear, colorless
suitability
suitable for molecular biology
UniProt accession no.
shipped in
ambient
storage temp.
−20°C
SMILES string
Cl[H].[H]N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](C(C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc4ccc(O)cc4)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc5ccccc5)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc6c[nH]c7ccccc67)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)O)C(O)=O
InChI
1S/C153H225N43O49S.ClH/c1-72(2)52-97(133(226)176-96(47-51-246-11)132(225)184-104(60-115(159)209)143(236)196-123(78(10)203)151(244)245)179-137(230)103(58-83-64-167-89-29-19-18-28-87(83)89)183-131(224)95(43-46-114(158)208)177-148(241)120(74(5)6)194-141(234)101(54-79-24-14-12-15-25-79)182-138(231)105(61-117(211)212)185-130(223)94(42-45-113(157)207)171-124(217)75(7)170-127(220)91(31-22-49-165-152(160)161)172-128(221)92(32-23-50-166-153(162)163)174-146(239)110(69-199)191-140(233)107(63-119(215)216)186-134(227)98(53-73(3)4)178-135(228)99(56-81-33-37-85(204)38-34-81)180-129(222)90(30-20-21-48-154)173-145(238)109(68-198)190-136(229)100(57-82-35-39-86(205)40-36-82)181-139(232)106(62-118(213)214)187-147(240)111(70-200)192-150(243)122(77(9)202)195-142(235)102(55-80-26-16-13-17-27-80)188-149(242)121(76(8)201)193-116(210)66-168-126(219)93(41-44-112(156)206)175-144(237)108(67-197)189-125(218)88(155)59-84-65-164-71-169-84;/h12-19,24-29,33-40,64-65,71-78,88,90-111,120-123,167,197-205H,20-23,30-32,41-63,66-70,154-155H2,1-11H3,(H2,156,206)(H2,157,207)(H2,158,208)(H2,159,209)(H,164,169)(H,168,219)(H,170,220)(H,171,217)(H,172,221)(H,173,238)(H,174,239)(H,175,237)(H,176,226)(H,177,241)(H,178,228)(H,179,230)(H,180,222)(H,181,232)(H,182,231)(H,183,224)(H,184,225)(H,185,223)(H,186,227)(H,187,240)(H,188,242)(H,189,218)(H,190,229)(H,191,233)(H,192,243)(H,193,210)(H,194,234)(H,195,235)(H,196,236)(H,211,212)(H,213,214)(H,215,216)(H,244,245)(H4,160,161,165)(H4,162,163,166);1H/t75-,76+,77+,78+,88-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-;/m0./s1
InChI key
RKGLLHCSSVJTAN-YYICOITRSA-N
Gene Information
human ... GCG(2641)
Looking for similar products? Visit Product Comparison Guide
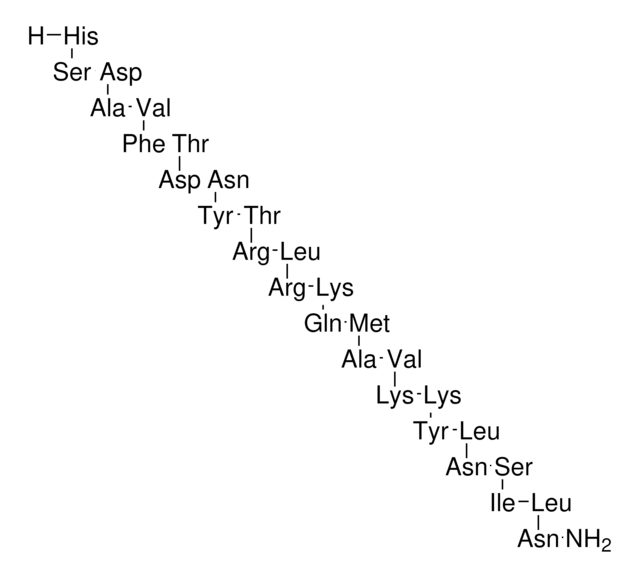
Amino Acid Sequence
General description
Application
- used as a component of hormone stock solution for preserving full biological activity of heart tissues obtained from male Sprague-Dawley rats
- used as an infusion in phases II and III fasting king penguins to study the various lipolytic, metabolic, and hormonal responses
- used for the stimulation of PGC-1α expression in hepatocytes
- used to induce the expression of methionine adenosyltransferase α1 (MAT1A), which is involved in the regulation of hepatic levels of S-adenosylmethionine and the adaptive response to fasting
- used to study its effect on stimulation of gluconeogenesis through hepatic lipolysis, mediated by inositol trisphosphate receptor 1 (INSP3R1)
- intraperitoneally injected in mice to assess glucagon-induced Sam68 subcellular localization in vivo
Biochem/physiol Actions
Components
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[Arg8]-Vasopressin solution Grade VI (synthetic), ~100 IU/mL in 0.9% NaCl](/deepweb/assets/sigmaaldrich/product/structures/326/242/dede8c26-cf73-4a28-a5d9-1d57c673cf0e/640/dede8c26-cf73-4a28-a5d9-1d57c673cf0e.png)