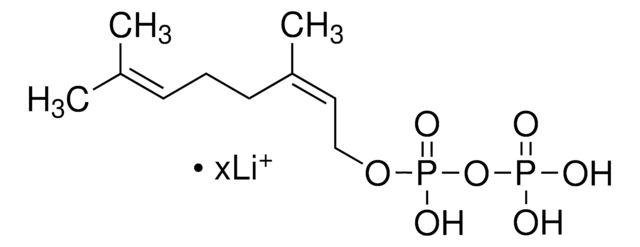
G6025
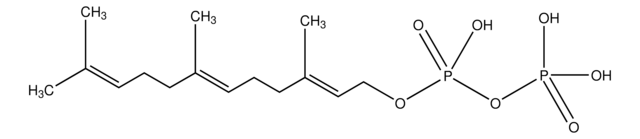
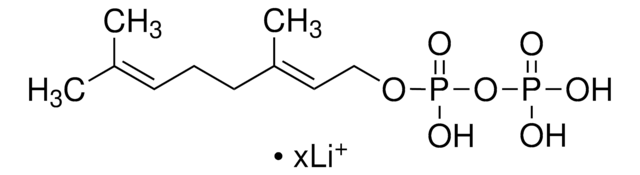
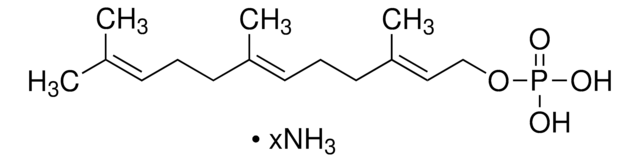
Geranylgeranyl pyrophosphate ammonium salt
solution, ≥95% (TLC), ~1 mg/mL in methanol: NH4OH (7:3)
Synonym(s):
GGPP, all trans-3,7,11,15-Tetramethyl-2,6,10,14-hexadecatetraenyl pyrophosphate ammonium salt
About This Item
Recommended Products
Assay
≥95% (TLC)
form
solution
packaging
vial of 200 μg
concentration
~1 mg/mL in methanol: NH4OH (7:3)
storage temp.
−20°C
SMILES string
C\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\COP(O)(=O)OP(O)(O)=O
InChI
1S/C20H36O7P2/c1-17(2)9-6-10-18(3)11-7-12-19(4)13-8-14-20(5)15-16-26-29(24,25)27-28(21,22)23/h9,11,13,15H,6-8,10,12,14,16H2,1-5H3,(H,24,25)(H2,21,22,23)/b18-11+,19-13+,20-15+
InChI key
OINNEUNVOZHBOX-QIRCYJPOSA-N
Application
- in the preparation of 2.5X reaction buffer for Rab GGTase assay
- to add to the culture to further drive Hmg2 degradation
- as a substrate to carryout in vitro enzymatic assays for the characterization of the prenyltransferases
- to reverse the action of lovastatin and also used to examine the potential role of GGPP in the study to assess the effects of exposing the melanoma cell lines in the angiogenesis model as a co-culture to lovastatin in vitro
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
51.8 °F - closed cup
Flash Point(C)
11 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service