L2251
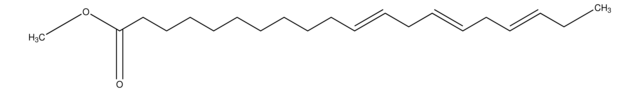
Methyl linolelaidate
Synonym(s):
Linolelaidic acid methyl ester, Methyl trans,trans-9,12-octadecadienoate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C19H34O2
CAS Number:
Molecular Weight:
294.47
Beilstein:
1727615
EC Number:
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
lipid type
unsaturated FAs
SMILES string
CCCCC\C=C\C\C=C\CCCCCCCC(=O)OC
InChI
1S/C19H34O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19(20)21-2/h7-8,10-11H,3-6,9,12-18H2,1-2H3/b8-7+,11-10+
InChI key
WTTJVINHCBCLGX-ZDVGBALWSA-N
Looking for similar products? Visit Product Comparison Guide
Packaging
Sealed ampule
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Effect of emeriamine, an inhibitor of fatty acid oxidation, on metabolic fate of a geometrical isomer of linoleic acid in perfused rat liver.
Fukuda N, Fukui M, Kai Y, Jayasooriya AP, et al.
J. Nutr. Sci. Vitaminol., 44, 525-535 (1998)
B M Craven et al.
Journal of lipid research, 24(6), 784-789 (1983-06-01)
At 123 K, crystals of cholesteryl trans-9-trans-12-octadecadienoate (cholesteryl linolelaidate, C47H76O2) are monoclinic, space group P2(1) with cell dimensions a = 13.03(3), b = 8.76(2), c = 17.90(4) A, beta = 89.7(2) degrees, having two molecules per unit cell. The crystal
T H Fischer et al.
Biochimica et biophysica acta, 1022(2), 215-228 (1990-02-28)
Arachidonate, at concentrations up to 50 microM, induced dose-dependent calcium efflux from preloaded microsomes prepared from human platelets, but not from unilamellar egg phosphatidylcholine vesicles. Arachidonate-induced efflux from microsomes was not inhibited by indomethacin, 13-azaprostanoic acid, or catalase and superoxide
Akio Kadowaki et al.
Chemistry and physics of lipids, 165(2), 178-185 (2012-01-03)
The antioxidative action of fullerenes has received much attention, but their reaction mechanism toward lipid-derived peroxyl radicals has not been well elucidated. In this study, the reaction products of [60]fullerene (C(60)) during the autoxidation of methyl linoleate (MeL) were isolated
Jian-Ying Feng et al.
European journal of medicinal chemistry, 46(4), 1198-1206 (2011-02-19)
In order to clarify the contribution of phenolic and enolic hydroxyl group to the antioxidant capacity of feruloylacetone, a model compound of half-curcumin, 6-(p-hydroxy-m-methoxyphenyl)-5-hexene-2,4-dione (FT), 6-(p-benzyloxy-m-methoxyphenyl)-5-hexene-2,4-dione (BMFT), 6-(m,p-dihydroxyphenyl)-5-hexene-2,4-dione (DDFT), 6-(p-hydroxy-m-methoxyphenyl)hexane-2,4-dione (DHFT), 6-(p-hydroxy-m-methoxyphenyl)-5-hexene-2,4-diol (THFT), and ethyl 2-(p-hydroxy-m-methoxybenzylidene)-3-oxobutanoate (EOFT) were synthesized. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service