L6785
Lactacystin
≥90% (HPLC), powder, proteosome inhibitor
About This Item
Recommended Products
product name
Lactacystin, ≥90% (HPLC)
Quality Level
Assay
≥90% (HPLC)
form
powder
potency
4 nM Ki (proteasome inhibitor)
solubility
water: 10 mg/mL, clear, colorless
storage temp.
−20°C
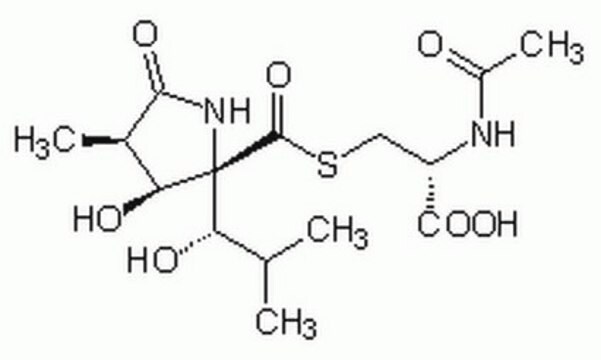
SMILES string
CC(C)[C@H](O)[C@]1(NC(=O)[C@H](C)[C@@H]1O)C(=O)SC[C@H](NC(C)=O)C(O)=O
InChI
1S/C15H24N2O7S/c1-6(2)10(19)15(11(20)7(3)12(21)17-15)14(24)25-5-9(13(22)23)16-8(4)18/h6-7,9-11,19-20H,5H2,1-4H3,(H,16,18)(H,17,21)(H,22,23)/t7-,9+,10+,11+,15-/m1/s1
InChI key
DAQAKHDKYAWHCG-RWTHQLGUSA-N
General description
Application
- as a proteasome inhibitor to inhibit protein degradation
- to inhibit proteasomal activity of cells for live cell imaging
- to block proteasomal proteolysis in human monocyte-derived dendritic cells (MoDCs) for 24 h
- to provide unilateral injection to animals to induce nigrostriatal lesions
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Discover definitions and uses for irreversible inhibitors including the types of irreversible inhibitors: suicide inhibitors, heavy metal inhibitors, and time-dependent inhibitors.
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Related Content
Select different protease inhibitor types based on your needs to prevent protein degradation during isolation and characterization and safeguard proteins in sample prep.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service