M4267
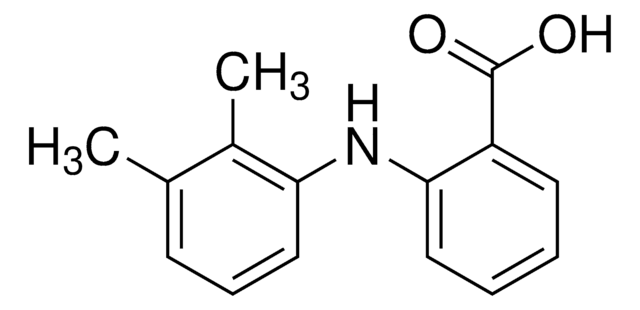
Mefenamic acid
Synonym(s):
2-[(2,3-Dimethylphenyl)amino]benzoic acid, N-(2,3-Xylyl)anthranilic acid
About This Item
Recommended Products
originator
Shionogi
SMILES string
Cc1cccc(Nc2ccccc2C(O)=O)c1C
InChI
1S/C15H15NO2/c1-10-6-5-9-13(11(10)2)16-14-8-4-3-7-12(14)15(17)18/h3-9,16H,1-2H3,(H,17,18)
InChI key
HYYBABOKPJLUIN-UHFFFAOYSA-N
Gene Information
human ... PTGS1(5742) , PTGS2(5743)
Looking for similar products? Visit Product Comparison Guide
Application
- to test its hepatotoxic effect in the transgenic zebrafish cell line (LiPan)
- to test its neuroprotective functionality in zebrafish embryos/larvae
- in the preparation of mucoadhesive microparticles in hydrogel beads
Biochem/physiol Actions
Features and Benefits
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Protein-based drug transporters are expressed in Sf9 cells. Understanding the specific mechanisms of tumor cell transporters is an essential aspect of chemotherapeutic drug design.
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service