R7257
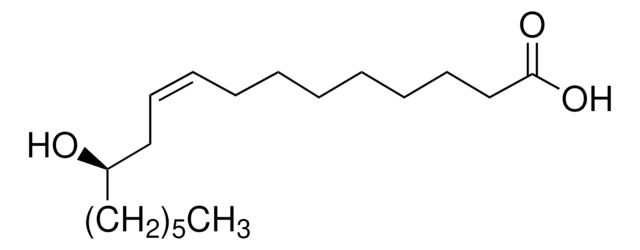
Ricinoleic acid
≥95%
Synonym(s):
Ricinelaidic acid, (R)-12-Hydroxy-cis-9-octadecenoic acid, 12-Hydroxyoleic acid
About This Item
Recommended Products
biological source
natural (organic)
Quality Level
Assay
≥95%
form
liquid
density
0.940 g/mL at 20 °C (lit.)
functional group
carboxylic acid
hydroxyl
lipid type
unsaturated FAs
shipped in
ambient
storage temp.
−20°C
SMILES string
CCCCCC[C@@H](O)C\C=C/CCCCCCCC(O)=O
InChI
1S/C18H34O3/c1-2-3-4-11-14-17(19)15-12-9-7-5-6-8-10-13-16-18(20)21/h9,12,17,19H,2-8,10-11,13-16H2,1H3,(H,20,21)/b12-9-/t17-/m1/s1
InChI key
WBHHMMIMDMUBKC-QJWNTBNXSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Multiplexed PLGA scaffolds with nitric oxide-releasing zinc oxide and melatonin-modulated extracellular vesicles for severe chronic kidney disease.: This innovative study utilizes ricinoleic acid in the fabrication of multiplexed PLGA scaffolds, demonstrating its effectiveness in enhancing the therapeutic efficacy of treatments for chronic kidney disease. The research underscores the potential of ricinoleic acid in medical applications, particularly in regenerative medicine and drug delivery systems (Rhim WK et al., 2024).
- Optimizing the enzymatic production of biolubricants by the Taguchi method: Esterification of the free fatty acids from castor oil with 2-ethyl-1-hexanol catalyzed by Eversa Transform 2.0.: Demonstrates the industrial application of ricinoleic acid in producing biolubricants, emphasizing its economic and environmental benefits. The study provides insights into the sustainable production processes and industrial applications of ricinoleic acid (Monteiro RRC et al., 2024).
Packaging
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
435.2 °F - closed cup
Flash Point(C)
224 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service