1604803
USP
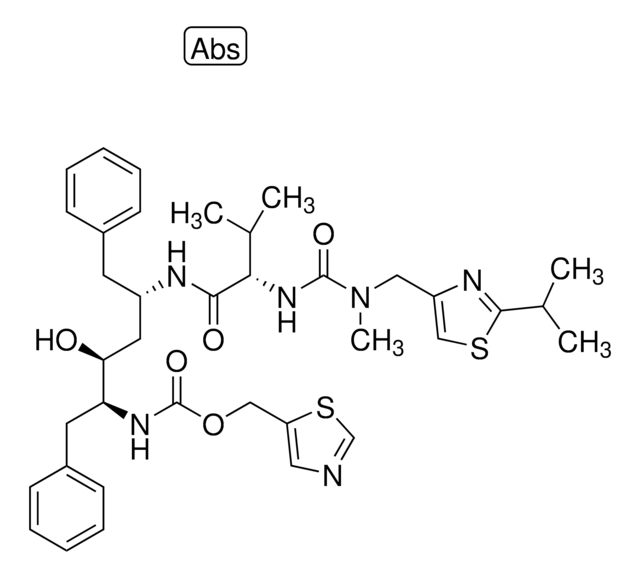
Ritonavir
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
1,3-Thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[[(2S)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,6-diphenylhexan-2-yl]carbamate
About This Item
Recommended Products
biological source
synthetic
grade
pharmaceutical primary standard
Agency
USP
vapor pressure
<0.0000001 kPa ( 25 °C)
API family
ritonavir
form
powder
packaging
pkg of 200 mg
manufacturer/tradename
USP
storage condition
protect from light
color
white to tan
solubility
acetonitrile: slightly soluble
ethanol: freely soluble
methanol: freely soluble
methylene chloride: freely soluble
water: practically insoluble
application(s)
pharmaceutical (small molecule)
format
neat
InChI
1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1
InChI key
NCDNCNXCDXHOMX-XGKFQTDJSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ritonavir belongs to the group of protease inhibitors that are widely used in combination with other drugs in the prevention of HIV. Its mode of action involves binding to the active site of the protease enzyme and preventing the further maturation of new viral particles.
Application
- Ritonavir Capsules
- Ritonavir Tablets
- Ritonavir Oral Solution
- Lopinavir and Ritonavir Tablets
- Lopinavir and Ritonavir Oral Solution
Analysis Note
Other Notes
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service