All Photos(1)
About This Item
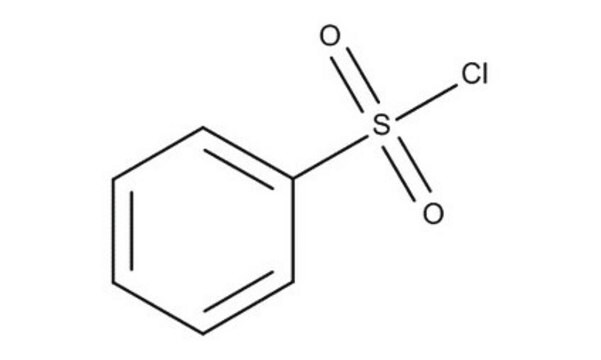
Linear Formula:
C6H5SO2Cl
CAS Number:
Molecular Weight:
176.62
Beilstein:
606926
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.04 mmHg ( 20 °C)
Quality Level
Assay
96%
refractive index
n20/D 1.551 (lit.)
bp
251-252 °C (lit.)
mp
13-15 °C (lit.)
solubility
alcohol: soluble
cold water: insoluble
diethyl ether: soluble
density
1.384 g/mL at 25 °C (lit.)
SMILES string
ClS(=O)(=O)c1ccccc1
InChI
1S/C6H5ClO2S/c7-10(8,9)6-4-2-1-3-5-6/h1-5H
InChI key
CSKNSYBAZOQPLR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzenesulfonyl chloride reacts with Grignard reagent from N-unsubstituted indoles to form oxindoles or substituted indoles. It is the derivatization reagent for the determination of various amines in waste water and surface water at the sub-ppb level by gas chromatography-mass spectrometry.
Application
Benzenesulfonyl chloride may be used in thiamine assay for determination of thiamine in different food products.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
260.6 °F
Flash Point(C)
127 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A G Soliman
Journal - Association of Official Analytical Chemists, 64(3), 616-622 (1981-05-01)
A semiautomated procedure was used to measure the fluorescence of sample extracts before and after the addition of benzenesulfonyl chloride (BSC). Addition of BSC inhibited thiochrome formation and provided a more representative blank based on the fluorescence of all the
Analysis of primary and secondary aliphatic amines in waste water and surface water by gas chromatography-mass spectrometry after derivatization with 2, 4-dinitrofluorobenzene or benzenesulfonyl chloride.
Sacher F, et al.
Journal of Chromatography A, 764(1), 85-93 (1997)
Unusual reactions of magnesium indolates with benzenesulfonyl chloride.
Wenkert E, et al.
The Journal of Organic Chemistry, 52(15), 3404-3409 (1987)
A Leone-Bay et al.
Journal of medicinal chemistry, 38(21), 4257-4262 (1995-10-13)
A series of benzoylated and phenylsulfonylated amino acids are novel, low molecular weight, self-assembling molecules. At low pH, these compounds form microspheres that dissolve readily under neutral conditions. In a given synthetic series, those molecules with low aqueous solubility formed
Ihor E Kopka et al.
Bioorganic & medicinal chemistry letters, 12(4), 637-640 (2002-02-15)
A series of substituted N-(3,5-dichlorobenzenesulfonyl)-L-prolyl- and alpha-methyl-L-prolyl-phenylalanine derivatives was prepared as VLA-4/VCAM antagonists. The compounds showed excellent potency with a wide variety of neutral, polar, electron withdrawing or donating groups on the phenylalanine ring (IC50 approximately 1 nM). Heteroaryl ring
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service