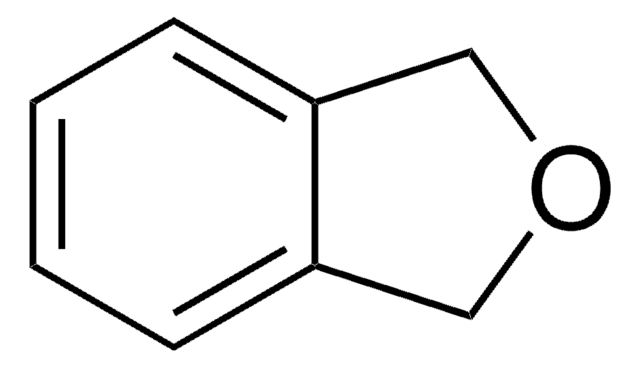
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H8O
CAS Number:
Molecular Weight:
120.15
Beilstein:
111928
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.549 (lit.)
bp
188-189 °C (lit.)
solubility
alcohol: soluble
carbon disulfide: soluble
chloroform: soluble
diethyl ether: soluble
density
1.065 g/mL at 25 °C (lit.)
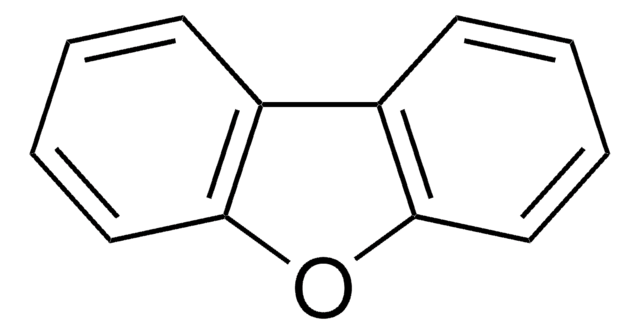
SMILES string
C1Cc2ccccc2O1
InChI
1S/C8H8O/c1-2-4-8-7(3-1)5-6-9-8/h1-4H,5-6H2
InChI key
HBEDSQVIWPRPAY-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
General description
Biotransformation of 2,3-dihydrobenzofuran using intact cells of Pseudomonas putida UV4 has been investigated. 2,3-Dihydrobenzofuran is the intermediate formed during catalytic hydrodeoxygenation of benzofuran.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
152.6 °F - closed cup
Flash Point(C)
67 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Catalytic hydrodeoxygenation of benzofuran and o-ethylphenol.
Lee C-L and Ollis DF.
J. Catal., 87(2), 325-331 (1984)
Xiangtai Meng et al.
Organic letters, 11(1), 137-140 (2008-12-05)
A new bifunctional phosphine catalyst, (2'-hydroxy-biphenyl-2-yl)-diethylphosphane (LBBA-1), was developed for the highly stereoselective synthesis of cis-2,3-dihydrobenzofurans via an aza-Morita-Baylis-Hillman/umpolung addition domino reaction of salicyl N-thiophosphinyl imines with electron-deficient allenes. Dual activation of both nucleophile and electrophile by the bifunctional catalyst
Guo-Hua Chu et al.
Bioorganic & medicinal chemistry letters, 15(23), 5114-5119 (2005-10-06)
Two novel chemical classes of kappa opioid receptor agonists, chroman-2-carboxamide derivatives and 2,3-dihydrobenzofuran-2-carboxamide derivatives, were synthesized. These agents exhibited high and selective affinity for the kappa opioid receptor.
Structures and stereochemical assignments of some novel chiral synthons derived from the biotransformation of 2, 3-dihydrobenzofuran and benzofuran by Pseudomonas putida.
Boyd DR, et al.
Tetrahedron Asymmetry, 4(6), 1307-1324 (1993)
Prashant P Deshpande et al.
Journal of industrial microbiology & biotechnology, 35(8), 901-906 (2008-05-23)
Microbial hydroxylation of o-bromophenylacetic acid provided 2-bromo-5-hydroxyphenylacetic acid. This enabled a route to the key intermediate 4-bromo-2,3-dihydrobenzofuran for synthesizing a melatonin receptor agonist and sodium hydrogen exchange compounds. Pd-mediated coupling reactions of 4-bromo-2,3-dihydrobenzofuran provided easy access to the 4-substituted-2,3-dihydrobenzofurans.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service