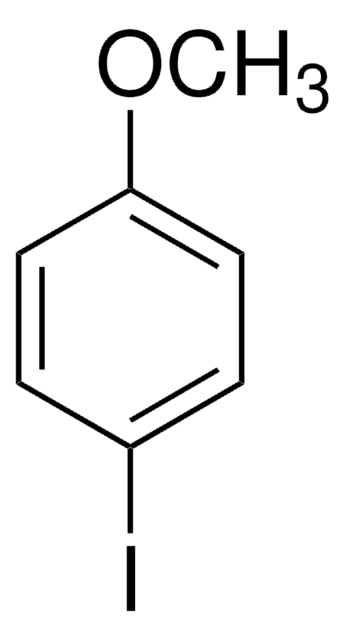
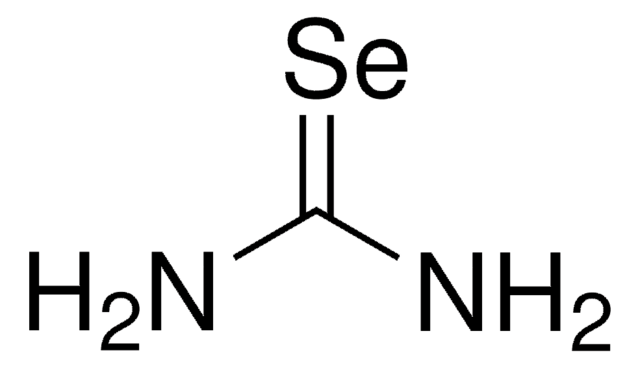
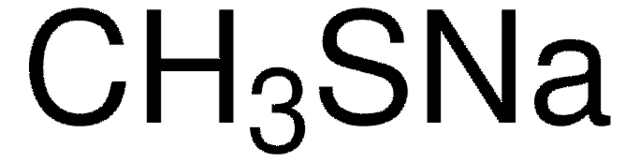367141
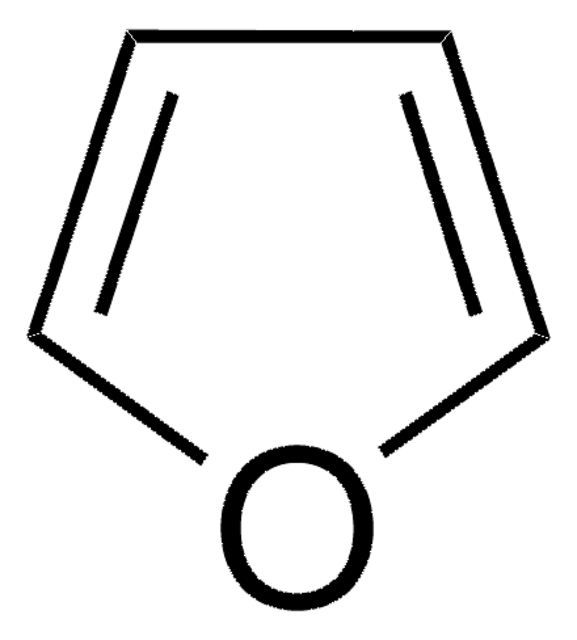
Selenophene
97%
Synonym(s):
Selenacyclopentadiene, Selenofuran
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H4Se
CAS Number:
Molecular Weight:
131.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.58 (lit.)
bp
110-111 °C (lit.)
mp
−30 °C (lit.)
density
1.423 g/mL at 25 °C (lit.)
SMILES string
c1cc[se]c1
InChI
1S/C4H4Se/c1-2-4-5-3-1/h1-4H
InChI key
MABNMNVCOAICNO-UHFFFAOYSA-N
General description
Selenophene is a heterocyclic building block. Synthesis of selenophene-based heteroacenes has been reported. Synthesis of selenophene-thiophene block copolymers has been reported. The electron transmission spectra of selenophene has been recorded in the 0-6eV energy range.
Application
Selenophene may be used:
- as conjugated linker in the synthesis of organic dyes
- in the synthesis of selenophene-2-carbonitrile
- in the synthesis of selenophene diketopyrrolopyrrole polymers
- as building block for the electrically conducting polyalkyl selenophene
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Liq. 2 - STOT RE 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
26.6 °F - closed cup
Flash Point(C)
-3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electron transmission spectra of selenophene and tellurophene and Xa computations of electron affinities for chalcophenes.
Modelli A, et al.
Chemical Physics, 88(3), 181-185 (1984)
Journal of the Electrochemical Society, 137, 1827-1827 (1990)
Low band gap selenophene-diketopyrrolopyrrole polymers exhibiting high and balanced ambipolar performance in bottom-gate transistors.
Shahid M, et al.
Chemical Science, 3(1), 181-185 (2012)
Dye-sensitized solar cells based on organic sensitizers with different conjugated linkers: furan, bifuran, thiophene, bithiophene, selenophene, and biselenophene.
Li R, et al.
The Journal of Physical Chemistry C, 113(17), 7469-7479 (2009)
Toshihiro Okamoto et al.
Organic letters, 7(23), 5301-5304 (2005-11-05)
[reaction: see text] A new intramolecular triple cyclization of bis(o-haloaryl)diacetylenes, via dilithiation followed by reaction with chalcogen elements, produces pi-conjugated compounds containing heterole-1,2-dichalcogenin-heterole fused tricyclic skeletons. The subsequent dechalcogenation with copper metal affords a series of thiophene- and selenophene-based heteroacenes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service