561657
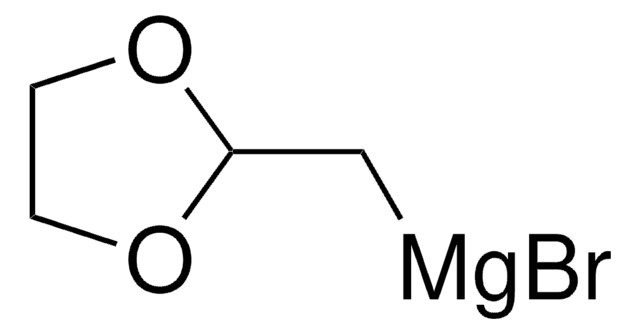
(1,3-Dioxan-2-ylethyl)magnesium bromide solution
0.5 M in THF
Synonym(s):
1,3-Dioxane - 2-ethyl- magnesium complex, [2-(1,3-Dioxan-2-yl)ethyl]magnesium bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11BrMgO2
CAS Number:
Molecular Weight:
219.36
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
reaction suitability
reaction type: Grignard Reaction
Quality Level
concentration
0.5 M in THF
bp
65 °C
density
0.951 g/mL at 25 °C
functional group
ether
storage temp.
2-8°C
SMILES string
Br[Mg]CCC1OCCCO1
InChI
1S/C6H11O2.BrH.Mg/c1-2-6-7-4-3-5-8-6;;/h6H,1-5H2;1H;/q;;+1/p-1
InChI key
JYNXRXBIEHSSLR-UHFFFAOYSA-M
Related Categories
Application
(1,3-Dioxan-2-ylethyl)magnesium bromide can be used:
- In a Grignard addition-acylation method for the preparation of enamides.
- To prepare trisubstituted allenes by reacting with propargylic ammonium salts.
- In one of the key synthetic steps for the synthesis of febrifugine based antimalarial drugs.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
No data available
Flash Point(C)
No data available
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Exploration of a new type of antimalarial compounds based on febrifugine.
Kikuchi H, et al.
Journal of Medicinal Chemistry, 49(15), 4698-4706 (2006)
Copper-catalysed cross-coupling of alkyl Grignard reagents and propargylic ammonium salts: stereospecific synthesis of allenes
Guisan-Ceinos M, et al.
Chemical Communications (Cambridge, England), 54(60), 8343-8346 (2018)
Oxonitriles: A Grignard Addition-Acylation Route to Enamides
Fleming FF, et al.
Organic Letters, 8(21), 4903-4906 (2006)
Fraser F Fleming et al.
Organic letters, 8(21), 4903-4906 (2006-10-06)
[reaction: see text] Sequential addition of three different Grignard reagents and pivaloyl chloride to 3-oxo-1-cyclohexene-1-carbonitrile installs four new bonds to generate a diverse array of cyclic enamides. Remarkably, formation of the C-magnesiated nitrile intermediate is followed by preferential acylation by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service