76763
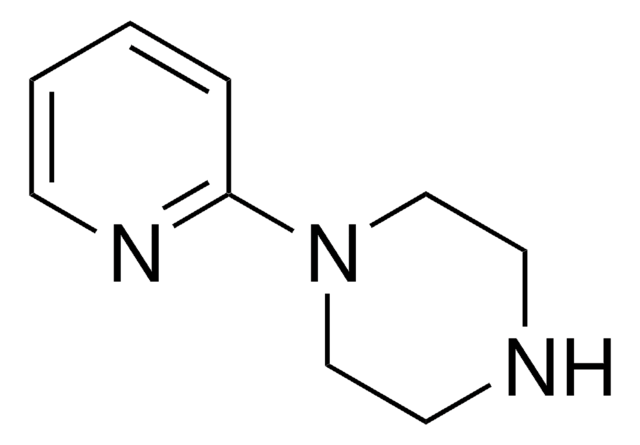
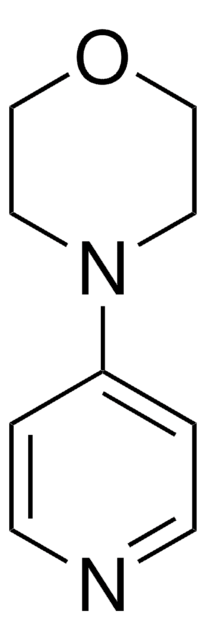
1-(4-Pyridyl)piperazine
≥97.0% (GC)
Synonym(s):
4-Piperazinopyridine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H13N3
CAS Number:
Molecular Weight:
163.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
form
powder
impurities
≤1% water
mp
137-141 °C
SMILES string
C1CN(CCN1)c2ccncc2
InChI
1S/C9H13N3/c1-3-10-4-2-9(1)12-7-5-11-6-8-12/h1-4,11H,5-8H2
InChI key
OQZBAQXTXNIPRA-UHFFFAOYSA-N
Gene Information
rat ... Chrnb2(54239)
Related Categories
General description
1-(4-Pyridyl) piperazine (or 4-Piperazinopyridine) is an active structural component that is used as a building block to prepare various medicinally important active molecules.
Application
1-(4-Pyridyl) piperazine can be used as a building block for the synthesis of:
- Nocathiacin I analogs for antibacterial studies.
- 4-amino-pyridyl derivatives, benzimido isoquinoline based derivatives and tert-pentylphenoxyalkyl piperazine derivatives for various biological applications.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, characterization, and bioactivity of new bisamidrazone derivatives as possible anticancer agents.
Al-Qtaitat M A, et al.
Medicinal Chemistry Research, 27(5), 1419-1431 (2018)
N ε-Acryloyllysine Piperazides as Irreversible Inhibitors of Transglutaminase 2: Synthesis, Structure?Activity Relationships, and Pharmacokinetic Profiling.
Wodtke R, et al.
Journal of Medicinal Chemistry, 61(10), 4528-4560 (2018)
Polymethacrylates containing a 4-amino-pyridyl derivative covalently attached as effective catalysts in acylation chemistry: self-activation by neighboring group effects
Mennenga T, et al.
Polymer International, 64(12), 1685-1689 (2015)
Benzimidazo [2, 1-a] benz [de] isoquinoline-7-one-12-carboxylic acid based fluorescent sensors for pH and Fe3+
Zhao Y, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 314(12), 52-59 (2016)
Selenium dioxide-mediated synthesis of ?-ketoamides from arylglyoxals and secondary amines
Shaw AY, et al.
Tetrahedron Letters, 53(32), 4151-4153 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service