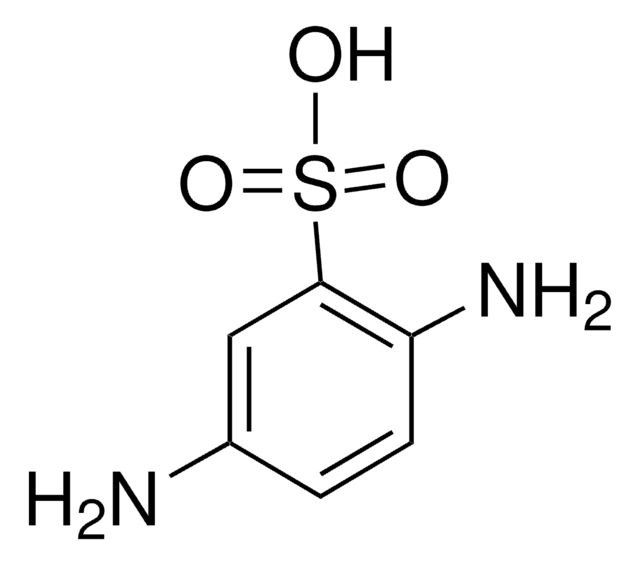
A86805
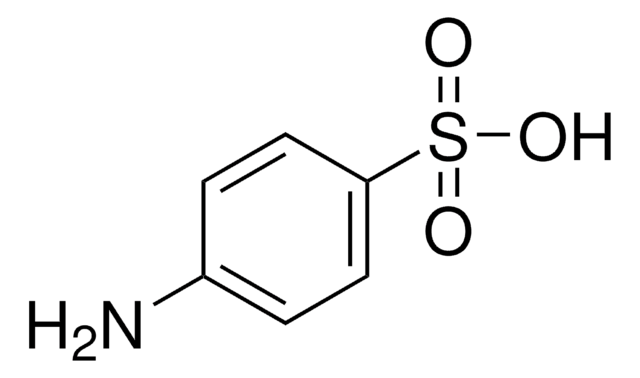
Aniline-2-sulfonic acid
95%
Synonym(s):
Orthanilic acid, 2-Aminobenzenesulfonic acid, o-Sulfanilic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H4SO3H
CAS Number:
Molecular Weight:
173.19
Beilstein:
1309204
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
SMILES string
Nc1ccccc1S(O)(=O)=O
InChI
1S/C6H7NO3S/c7-5-3-1-2-4-6(5)11(8,9)10/h1-4H,7H2,(H,8,9,10)
InChI key
ZMCHBSMFKQYNKA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xin-Gui Li et al.
Journal of combinatorial chemistry, 8(2), 174-183 (2006-03-15)
A unique strategy for synthesis of narrowly distributed and inherently self-stabilized copolymer nanoparticles by a simple emulsifier-free polymerization from orthanilic acid and aniline was developed. The polymerization yield, electrical conductivity, size, and its distribution of the nanoparticles could be simultaneously
Jürgen Ruff et al.
Microbiological research, 165(4), 288-299 (2009-07-07)
Alcaligenes sp. strain O-1 inducibly deaminates 2-aminobenzenesulfonate (ABS) via dioxygenation to 3-sulfocatechol, which is desulfonated during meta ring-cleavage to yield 2-hydroxymuconate. This intermediate is transformed through the oxalocrotonate-branch of the sulfocatechol meta-pathway (Scm). The complete pathway is encoded on the
Determination of nitrite ion and sulfanilic and orthanilic acids by differential pulse polarography.
S T Sulaiman
Analytical chemistry, 56(13), 2405-2407 (1984-11-01)
F Junker et al.
The Biochemical journal, 300 ( Pt 2), 429-436 (1994-06-01)
2-Aminobenzenesulphonic acid (2AS) is degraded by Alcaligenes sp. strain O-1 via a previously detected but unidentified intermediate. A mutant of strain O-1 was found to excrete this intermediate, which was isolated and identified by m.s., 1H- and 13C-n.m.r. as 3-sulphocatechol
Inotropic activity of orthanilic and L-cysteic acid on isolated guinea-pig ventricular strips.
F Franconi et al.
Advances in experimental medicine and biology, 217, 159-165 (1987-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service