B56501
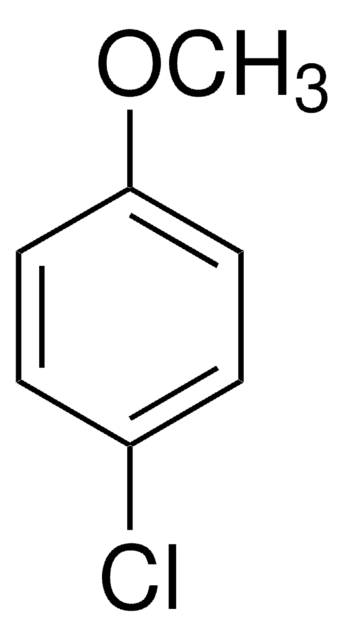
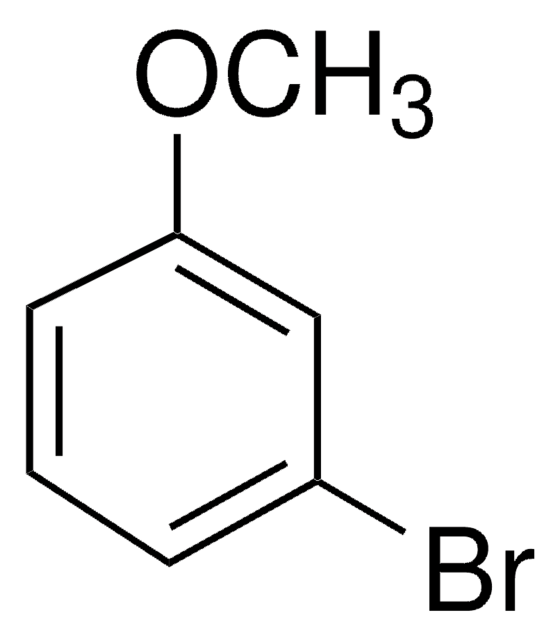
4-Bromoanisole
≥99.0%
Synonym(s):
1-Bromo-4-methoxybenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4OCH3
CAS Number:
Molecular Weight:
187.03
Beilstein:
1237590
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0%
form
liquid
refractive index
n20/D 1.564 (lit.)
bp
223 °C (lit.)
mp
9-10 °C (lit.)
density
1.494 g/mL at 25 °C (lit.)
SMILES string
COc1ccc(Br)cc1
InChI
1S/C7H7BrO/c1-9-7-4-2-6(8)3-5-7/h2-5H,1H3
InChI key
QJPJQTDYNZXKQF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
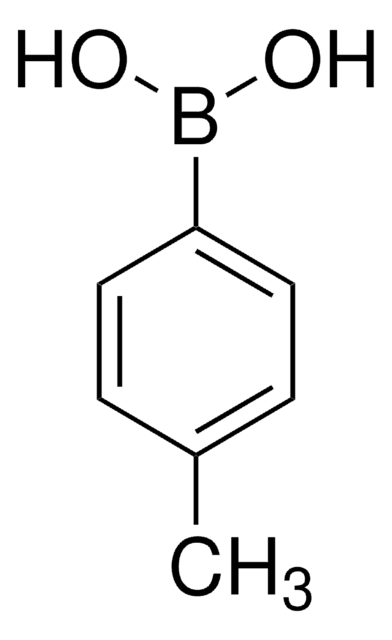
4-Bromoanisole is a useful brominating reagent. It is formed as reaction product in the reaction between HOBr and anisole. Suzuki coupling of 4-bromoanisole with phenylboronic acid catalyzed by palladium pincer complexes has been studied. Heck Reaction of 4-bromoanisole with ethyl acrylates in room-temperature ionic liquids is reported to afford ethyl 4-methoxycinnamate.
Application
4-Bromoanisole was used in the synthesis of aryl 1,3-diketones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
201.2 °F
Flash Point(C)
94 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amy E Bryant et al.
PloS one, 12(2), e0172486-e0172486 (2017-03-01)
Acute muscle injuries are exceedingly common and non-steroidal anti-inflammatory drugs (NSAIDs) are widely consumed to reduce the associated inflammation, swelling and pain that peak 1-2 days post-injury. While prophylactic use or early administration of NSAIDs has been shown to delay
Dennis U Nielsen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(52), 17926-17938 (2013-11-23)
Reaction conditions for the three-component synthesis of aryl 1,3-diketones are reported applying the palladium-catalyzed carbonylative α-arylation of ketones with aryl bromides. The optimal conditions were found by using a catalytic system derived from [Pd(dba)2] (dba=dibenzylideneacetone) as the palladium source and
Palladium bis (phosphinite)'PCP'-pincer complexes and their application as catalysts in the Suzuki reaction.
Bedford RB, et al.
New. J. Chem., 24(10), 745-747 (2000)
Grace E Solini et al.
Developmental biology, 460(2), 99-107 (2020-01-04)
As an essential feature of development, robustness ensures that embryos attain a consistent phenotype despite genetic and environmental variation. The growing number of examples demonstrating that embryos can mount a compensatory response to germline mutations in key developmental genes has
Charles E Wood et al.
Carcinogenesis, 36(7), 782-791 (2015-04-29)
Environmental exposures occurring early in life may have an important influence on cancer risk later in life. Here, we investigated carryover effects of dichloroacetic acid (DCA), a small molecule analog of pyruvate with metabolic programming properties, on age-related incidence of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service