C100005
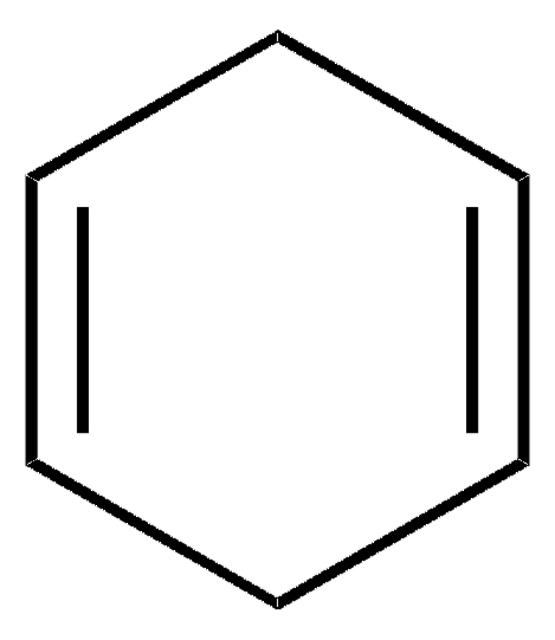
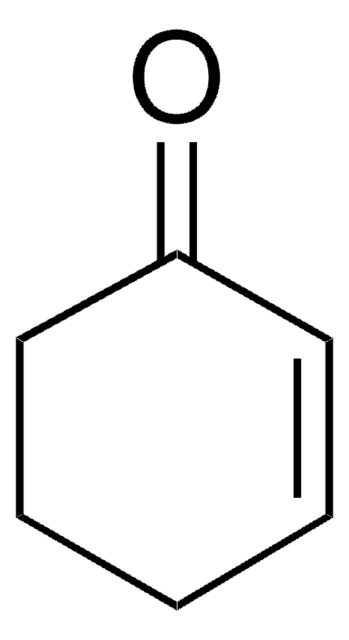
1,3-Cyclohexadiene
contains 0.05% BHT as inhibitor, 97%
Synonym(s):
1,2-Dihydrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8
CAS Number:
Molecular Weight:
80.13
Beilstein:
506024
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
contains
0.05% BHT as inhibitor
refractive index
n20/D 1.474 (lit.)
bp
80 °C (lit.)
density
0.841 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1CC=CC=C1
InChI
1S/C6H8/c1-2-4-6-5-3-1/h1-4H,5-6H2
InChI key
MGNZXYYWBUKAII-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,3-Cyclohexadiene can undergo:
- C-C coupling with aromatic alcohols via iridium-catalyzed hydrogen auto-transfer and with aldehydes via transfer hydrogenation mediated by isopropanol to form carbonyl addition products.
- Living anionic polymerization with n-BuLi/TMEDA system to form polycyclohexadiene.
- Platinum-catalyzed silaboration to form (1R,4S)-1-(dimethylphenylsilyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-cyclohexene.
- Aerobic palladium-catalyzed 1,4-diacetoxylation in the presence of cobalt tetra(hydroquinone)porphyrin as an electron transfer reagent.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
66.0 °F
Flash Point(C)
18.9 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cobalt Tetra (hydroquinone) porphyrin: An Efficient Electron Transfer Reagent in Aerobic Pd?Catalyzed 1, 4?Diacetoxylation of 1, 3?Cyclohexadiene.
Grennberg H, et al.
Angewandte Chemie (International Edition in English), 32(2), 263-264 (1993)
Nicolas J Saettel et al.
Journal of the American Chemical Society, 124(38), 11552-11559 (2002-09-19)
The electron-transfer-catalyzed Diels-Alder reaction of indole and 1,3-cyclohexadiene was studied by a combination of experimental and theoretical methods. The (13)C kinetic isotope effects were determined at natural abundance by NMR methodology. B3LYP/6-31G* calculations allow for the first time a quantitatively
Yasushi Obora et al.
The Journal of organic chemistry, 75(17), 6046-6049 (2010-08-05)
Intermolecular [2+2+2] cycloaddition of tert-butylacetylene with alpha,omega-dienes was successfully achieved by NbCl(3)(DME) catalyst to afford 5-omega-alkenyl-1,4-disubstituted-1,3-cyclohexadienes in excellent yields with high chemo- and regioselectivity.
Synthesis of polymers with an alicyclic structure in the main chain. Living anionic polymerization of 1, 3-cyclohexadiene with the n-butyllithium/N, N, N ′, N′-tetramethyl-ethylenediamine system.
Natori I.
Macromolecules, 30(12), 3696-3697 (1997)
Takumi Oshima et al.
Organic & biomolecular chemistry, 10(9), 1730-1734 (2012-01-13)
The first CH/π solute-solvent interaction of C(60) was evidenced by the kinetic solvent effects in the Diels-Alder reaction with 1,3-cyclohexadiene based on the evaluation of linear free energy relationship of log k(2) with empirical solvent polarity and basicity parameters, E(T)(30)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service