A6563
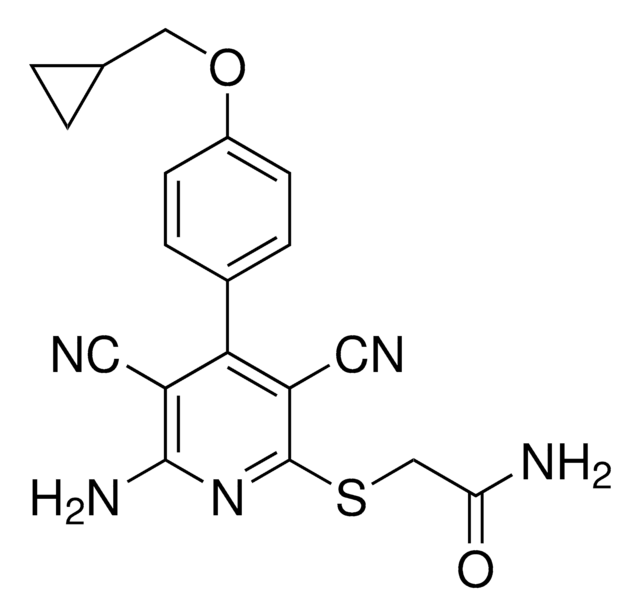
6-Anilinoquinoline-5,8-quinone
≥95% (TLC), solid
Synonym(s):
6-(Phenylamino)-5,8-quinolinedione, LY-83,583, LY83583
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H10N2O2
CAS Number:
Molecular Weight:
250.25
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥95% (TLC)
form
solid
color
violet
solubility
0.1 M HCl: 1 mg/mL
methanol: 11 mg/mL
ethanol: 8 mg/mL
storage temp.
2-8°C
SMILES string
O=C1C=C(Nc2ccccc2)C(=O)c3cccnc13
InChI
1S/C15H10N2O2/c18-13-9-12(17-10-5-2-1-3-6-10)15(19)11-7-4-8-16-14(11)13/h1-9,17H
InChI key
GXIJYWUWLNHKNW-UHFFFAOYSA-N
Application
6-Anilinoquinoline-5,8-quinone inhibits soluble guanylate cyclase and cGMP production. 6-Anilinoquinoline-5,8-quinone also blocks the release of intracellular Ca2+ and antigen-induced leukotrienes.
Biochem/physiol Actions
Blocks cGMP production; inhibits intracellular Ca2+ release; blocks the effects of nitric oxide. Inhibits antigen-induced leukotriene release.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R Hernanz et al.
Methods and findings in experimental and clinical pharmacology, 21(4), 243-251 (1999-07-10)
Bradykinin (BK) induced endothelium- and concentration-dependent relaxations in segments of porcine posterior descending coronary arteries submaximally precontracted with the thromboxane A2 mimetic, U-46619. The effects of BK were reduced by L-NG-monomethylarginine (L-NMMA) and 6-anilinoquinoline-5,8-quinone (LY-83583), respective inhibitors of nitric oxide
Ole De Backer et al.
European journal of pharmacology, 590(1-3), 369-376 (2008-07-08)
This study investigated the possible interaction between the heme oxygenase (HO)/biliverdin reductase (BVR) and nitric oxide synthase (NOS) pathway in murine gastric fundus and jejunum, since previous studies have shown that both HO-2 and BVR are expressed in interstitial cells
Yao Teng et al.
Journal of plant physiology, 167(11), 885-889 (2010-02-23)
Cyclic guanosine 3',5'-monophosphate (cGMP) is an important second messenger in animals, and is emerging as a player in regulatory functions in plants. In this study, we investigated the role of cGMP in seed germination in Arabidopsis thaliana (Col-0). We demonstrated
Márcia Regina Ribeiro et al.
The Biological bulletin, 216(2), 138-148 (2009-04-16)
The cell signaling cascades that mediate pigment movements in crustacean chromatophores are not yet well established, although Ca(2+) and cyclic nucleotide second messengers are involved. Here, we examine the participation of cyclic guanosine monophosphate (cGMP) in pigment aggregation triggered by
I Fleming et al.
British journal of pharmacology, 103(1), 1047-1052 (1991-05-01)
1. The aim of this investigation was to study the relationship between contractile responsiveness, activation of the L-arginine pathway and tissue levels of guanosine 3':5'cyclic monophosphate (cylic GMP) in aortic rings removed from rats 4 h after intraperitoneal administration of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Benz[g]isoquinoline-5,10-dione 99%](/deepweb/assets/sigmaaldrich/product/structures/484/029/288c4a9d-19c2-4b51-82c1-f43b50ea05b0/640/288c4a9d-19c2-4b51-82c1-f43b50ea05b0.png)