All Photos(3)
About This Item
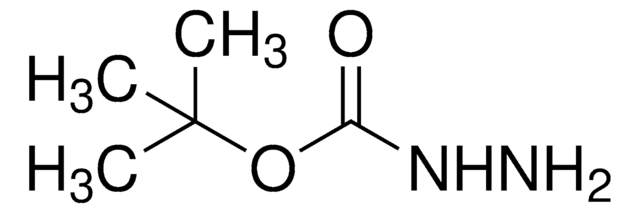
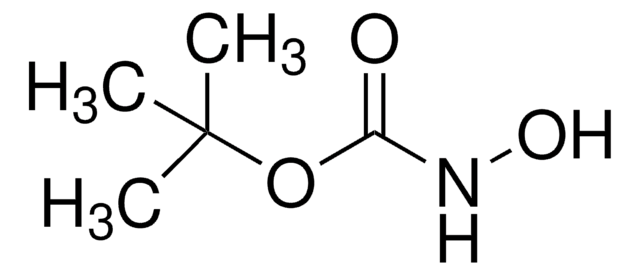
Linear Formula:
NH2COOC(CH3)3
CAS Number:
Molecular Weight:
117.15
Beilstein:
1744500
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
105-108 °C (lit.)
functional group
amine
SMILES string
CC(C)(C)OC(N)=O
InChI
1S/C5H11NO2/c1-5(2,3)8-4(6)7/h1-3H3,(H2,6,7)
InChI key
LFKDJXLFVYVEFG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Palladium-catalyzed cross-coupling reaction of tert-butyl carbamate with various aryl(Het) halides with Cs2CO3 as base in 1,4-dioxane (solvent) has been investigated.
Application
tert-Butyl carbamate was used in palladium-catalyzed synthesis of N-Boc-protected anilines. It was used in the synthesis of tetrasubstituted pyrroles, functionalized with ester or ketone groups at C-3 position.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maximilian Tromayer et al.
Polymer chemistry, 8(2), 451-460 (2017-03-07)
The possibility of the direct encapsulation of living cells
Pd-catalyzed amidation of aryl (Het) halides with< i> tert</i>-butyl carbamate.
Qin L, et al.
Tetrahedron Letters, 51(33), 4446-4448 (2010)
One-Pot Three-Component Synthesis of Tetrasubstituted NH Pyrroles from Secondary Propargylic Alcohols, 1, 3-Dicarbonyl Compounds and tert-Butyl Carbamate.
Cadierno V, et al.
Journal of Heterocyclic Chemistry, 47(1), 233-236 (2010)
Swapna Bhagwanth et al.
The Journal of organic chemistry, 74(12), 4634-4637 (2009-06-13)
The scope of Pd-catalyzed synthesis of N-Boc-protected anilines from aryl bromides and commercially available tert-butyl carbamate is described. For the first time, this process can be conducted at room temperature (17-22 degrees C) using a combination of Pd(2)dba(3).CHCl(3) and a
Tetsuo Cai et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 39(43), 8600-8610 (2019-09-19)
γ-Secretase is an intramembrane-cleaving protease that generates the toxic species of the amyloid-β peptide (Aβ) that is responsible for the pathology of Alzheimer disease. The catalytic subunit of γ-secretase is presenilin 1 (PS1), which is a polytopic membrane protein with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service