219991
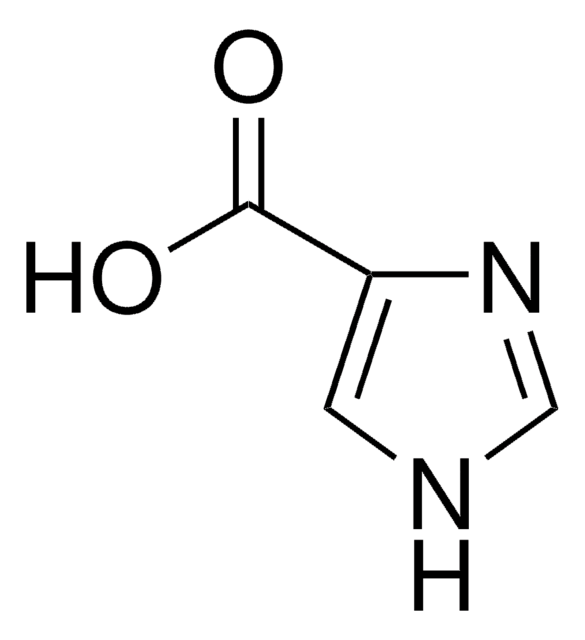
4-Imidazoleacetic acid hydrochloride
98%
Synonym(s):
(4-Imidazolyl)acetic acid hydrochloride, I4AA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6N2O2 · HCl
CAS Number:
Molecular Weight:
162.57
Beilstein:
3701591
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
218-222 °C (lit.)
solubility
water: soluble 100 mg/mL, clear, faintly yellow
functional group
carboxylic acid
SMILES string
Cl.OC(=O)Cc1c[nH]cn1
InChI
1S/C5H6N2O2.ClH/c8-5(9)1-4-2-6-3-7-4;/h2-3H,1H2,(H,6,7)(H,8,9);1H
InChI key
MWHLCFYPFGFBQO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Imidazoleacetic acid hydrochloride was used in the synthesis of:
- imidazolyl-polyethylenimine modified nanoparticles
- pyridyl and imidazoyl functionalized carboproteins, potential metal ion chelators
Biochem/physiol Actions
Competitive antagonist at GABAC receptors.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Archana Swami et al.
International journal of pharmaceutics, 335(1-2), 180-192 (2006-12-16)
The derivatives of polyethylenimine (PEI 25 and 750kDa) were synthesized by partially substituting their amino groups with imidazolyl moieties. The series of imidazolyl-PEIs thus obtained were cross-linked with polyethylene glycol (PEG) to get imidazolyl-PEI-PEG nanoparticles (IPP). The component of hydrophobicity
A Pernille Tofteng et al.
Organic & biomolecular chemistry, 5(14), 2225-2233 (2007-07-05)
De novo design and total chemical synthesis of proteins provides a powerful approach for biological and biophysical studies with the ability to prepare artificial proteins with tailored properties, potentially of importance for biophysical studies, material science, nanobioscience, and as molecular
Karin N Barnouin et al.
Proteomics, 5(17), 4376-4388 (2005-11-19)
IMAC can be used to selectively enrich phosphopeptides from complex peptide mixtures, but co-retention of acidic peptides together with the failure to retain some phosphopeptides restricts the general utility of the method. In this study Fe(III)-IMAC was qualitatively and quantitatively
Nan Yan et al.
Small (Weinheim an der Bergstrasse, Germany), 15(41), e1903016-e1903016 (2019-08-20)
Developing tumor-responsive diagnosis and therapy strategies for tumor theranostics is still a challenge owing to their high accuracy and specificity. Herein, an AND logic gated-DNA nanodevice, based on the fluorescence nucleic acid probe and polymer-modified MnO2 nanosheets, for glutathione (GSH)-gated
Theoretical studies of IMAC interfacial phenomena for the production of protein C.
E Eileen Thiessen et al.
Advances in experimental medicine and biology, 540, 183-190 (2004-06-04)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service