All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H11N
CAS Number:
Molecular Weight:
85.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.437 (lit.)
bp
97-99 °C/744 mmHg (lit.)
density
0.834 g/mL at 25 °C (lit.)
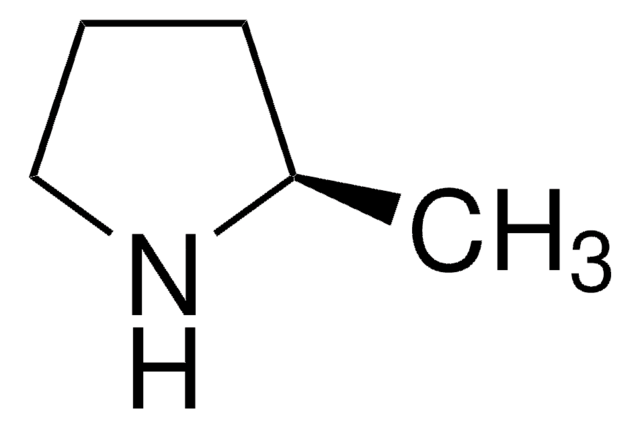
SMILES string
CC1CCCN1
InChI
1S/C5H11N/c1-5-3-2-4-6-5/h5-6H,2-4H2,1H3
InChI key
RGHPCLZJAFCTIK-UHFFFAOYSA-N
General description
2-Methylpyrrolidine is a substituted pyrrolidine. It is one of the major bioactive compounds found in Teucrium manghuaense. The hydrodenitrogenation of 2-methylpyrrolidine in the presence of sulfided NiMo/γ-Al2O3 catalyst has been studied.
Application
2-Methylpyrrolidine may be used as a guest molecule for the synthesis of nonasil-[4158], a clatharate compound.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
37.4 °F - closed cup
Flash Point(C)
3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jennifer Zablocki et al.
Chirality, 32(5), 619-631 (2020-03-11)
An enantiomerically pure (R)-2-methylpyrrolidine-based anilino squaraine crystallizes in two chiral polymorphs adopting a monoclinic C2 and an orthorhombic P21 21 21 structure, respectively. By various thin-film preparation techniques, a control of the polymorph formation is targeted. The local texture of
Extraction of Teucrium manghuaense and evaluation of the bioactivity of its extract.
Yin G, et al.
International Journal of Molecular Sciences, 10(10), 4330-4341 (2009)
Nagaraju Rajana et al.
Journal of chromatographic science, 57(9), 769-777 (2019-09-11)
Acetamide is a potential genotoxic impurity; it should control in drug substance based on daily dosage level. It forms from base-contaminated acetonitrile and by-product of some drug substances. The available methods for acetamide in drug substance and water samples were
On the Formation of Pentylpiperidine in the Hydrodenitrogenation of Pyridine.
Wang H and Prins R.
Catalysis Letters, 126(1-2), 1-9 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service