549606
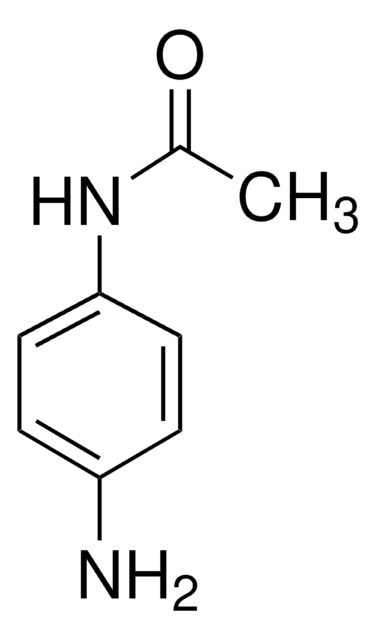
2′-Aminoacetanilide
98%
Synonym(s):
N-(2-Aminophenyl)acetamide, o-Aminoacetanilide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CONHC6H4NH2
CAS Number:
Molecular Weight:
150.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
mp
133-137 °C (lit.)
functional group
amide
SMILES string
CC(=O)Nc1ccccc1N
InChI
1S/C8H10N2O/c1-6(11)10-8-5-3-2-4-7(8)9/h2-5H,9H2,1H3,(H,10,11)
InChI key
MPXAYYWSDIKNTP-UHFFFAOYSA-N
General description
2′-Aminoacetanilide (2-aminoacetanilide, o-aminoacetanilide) can be prepared by the catalytic hydrogenation of 2-nitroacetanilide using 10%Pd/C (palladium/carbon).
Application
2′-Aminoacetanilide may be used in the preparation of:
- 2-Methylbenzimidazole.
- N-(2-(1,3-Dimethyl-2,4-dioxo-5-phenyl-3,4-dihydro-1H-pyrrolo[3,4-d]pyrimidin-6(2H)-yl)phenyl)-5-methylfuran-2-carboxamide, a potent cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor.
- Azobenzothiazole dyes, N-[2-(6-nitrobenzothiazol-2-ylazo)phenyl]acetamide and N-[2-(benzothiazol-2-ylazo)phenyl]acetamide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A short synthesis of phenanthro [2, 3-d] imidazoles from dehydroabietic acid. Application of the methodology as a convenient route to benzimidazoles.
Fonseca T, et al.
Tetrahedron, 57(9), 1793-1799 (2001)
Cyclization of o-phenylenediamines by CO2 in the presence of H2 for the synthesis of benzimidazoles.
Yu B, et al.
Green Chemistry, 15(1), 95-99 (2013)
Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model.
Tradtrantip L, et al.
Journal of Medicinal Chemistry, 52(20), 6447-6455 (2009)
Novel azobenzothiazole dyes from 2-nitrosobenzothiazoles.
Faustino H, et al.
Dyes and Pigments, 83(1), 88-94 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service