All Photos(1)
About This Item
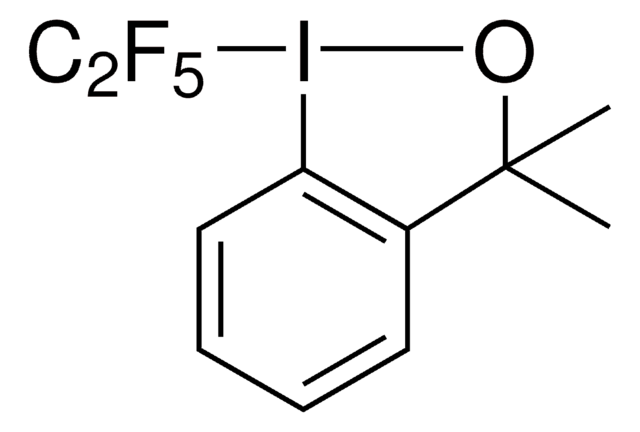
Linear Formula:
C8H5BrF4S
CAS Number:
MDL number:
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
form
liquid
reaction suitability
reaction type: C-C Bond Formation
Application
Phenylsulfanyltetrafluoroethyl bromide is a fluoroalkylbromide that is a radical source of the phenylsulfanyltetrafluoroethyl moiety. Alternatively, it can also be selectively metallated at the fluorinated carbon with Turbo Grignard reagent at low temperatures resulting in a thermally unstable anion that can act as a nucleophilic fluoroalkylation reagent towards a wide variety of electrophiles, such as aldehydes, ketones or sulfonylimines. A phenylsulfanyltetrafluoroethyl moiety incorporated in the substrate can be treated with tributyltin hydride and generate the corresponding fluoroalkyl radical. If the substrate lacks any olefins, it can be reduced to tetrafluoroethyl group whereas if the substrate contains an olefin in a correct spatial orientation, it can lead to an intramolecular cyclization affording tetrafluorinated cyclic structures.
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Legal Information
Product of CF Plus Chemicals.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Fluoroalkylation toolbox expands with various reagents for late-stage fluoroalkylation in organic synthesis and medicinal chemistry.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service