D116408
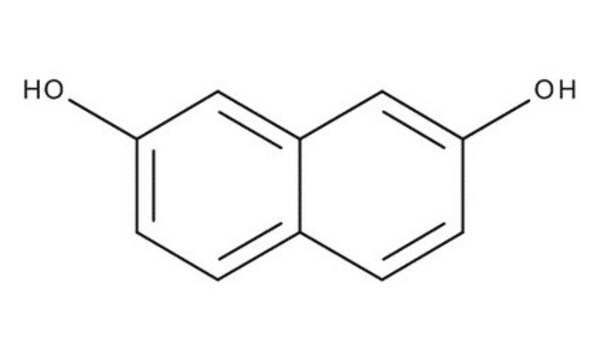
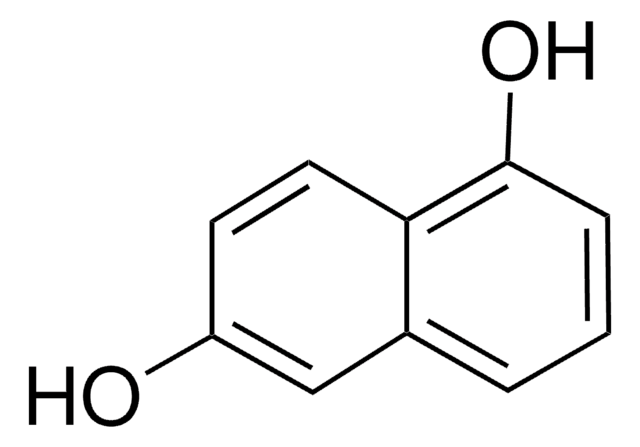
2,7-Dihydroxynaphthalene
97%
Synonym(s):
2,7-Naphthalenediol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C10H6(OH)2
CAS Number:
Molecular Weight:
160.17
Beilstein:
2042383
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
185-190 °C (lit.)
SMILES string
Oc1ccc2ccc(O)cc2c1
InChI
1S/C10H8O2/c11-9-3-1-7-2-4-10(12)6-8(7)5-9/h1-6,11-12H
InChI key
DFQICHCWIIJABH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,7-Dihydroxynaphthalene is a organic building block used to prepare sulfonic acids, divinylnaphthalenes, dyes, pigments, and fluorescent whiteners.
Application
Starting material for the synthesis of sulfonic acids and divinylnaphthalenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Allergic contact dermatitis from 2,7-dihydroxynaphthalene in hair dye.
A Eskelinen et al.
Contact dermatitis, 36(6), 312-313 (1997-06-01)
Alexandru Chichirau et al.
Free radical biology & medicine, 38(3), 344-355 (2005-01-05)
ortho-Hydroxyphenols (catechols) form a common structural unit in naturally occurring antioxidants such as polyphenols. They also show pro-oxidant characteristics which depend on their particular structure. Here we examined the acetylated versions of three catechols and a naphthalenediol for cytotoxicity to
Elisa Haug-Schifferdecker et al.
The Journal of biological chemistry, 285(22), 16487-16494 (2010-03-31)
Five fungal genomes from the Ascomycota (sac fungi) were found to contain a gene with sequence similarity to a recently discovered small group of bacterial prenyltransferases that catalyze the C-prenylation of aromatic substrates in secondary metabolism. The genes from Aspergillus
Efficient lactic acid-catalyzed route to naphthopyranopyrimidines under solvent-free conditions
Sadeh FN, et al.
Organic preparations and procedures international, 49, 35-44 (2017)
Raafat M Issa et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 62(4-5), 980-986 (2005-06-14)
The absorption spectra of mono- and bis-azo-derivatives obtained by coupling the diazonium salts of aromatic amines and 2,7-dihydroxynaphthalene have been studied in six organic solvents. The different absorption bands have been assigned and the effect of solvents on the charge
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service