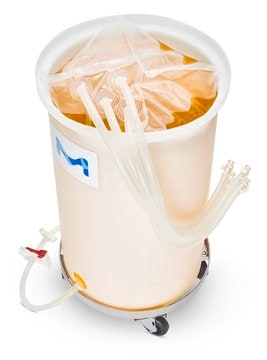
SAU0200L41DP5
Mobius® Silver Drum Assembly
200L Standard PureFlex™ Drum Assembly
Synonym(s):
Mobius®, Silver, 200 L Standard PureFlex™ Drum Assembly
About This Item
Recommended Products
material
PureFlex™ container
Quality Level
product line
EMPROVE® Single Use
Mobius®
parameter
200 L sample volume
Looking for similar products? Visit Product Comparison Guide
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Key aspects of single-use assembly qualification including quality by design (QbD), quality risk management (QRM) and operator handling and training.
Before adoption of single-use technologies in biomanufacturing, manufacturers must assess the risk to the drug product from potential leachables. This article highlights a general approach based on the United States Pharmacopeia (USP).
This page provides an overview of USP <87>, <88>, and <1031>, compares bioreactivity testing, and describes the transition of our organization towards in vitro test methods.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service