07672
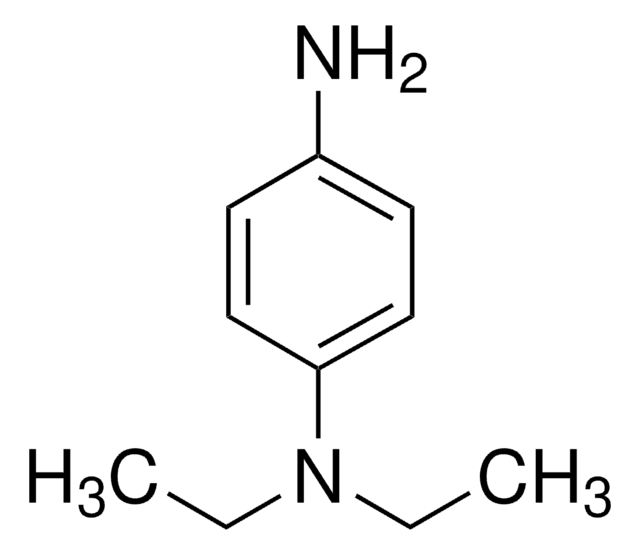
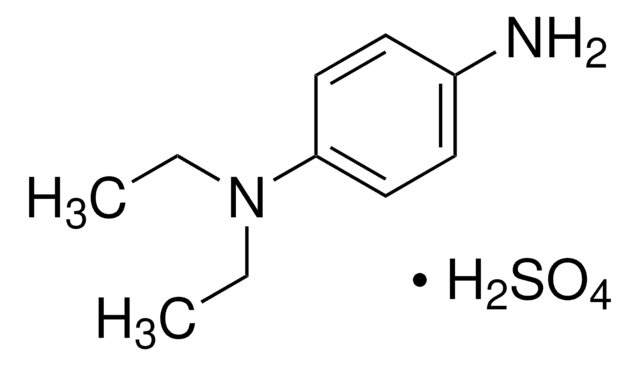
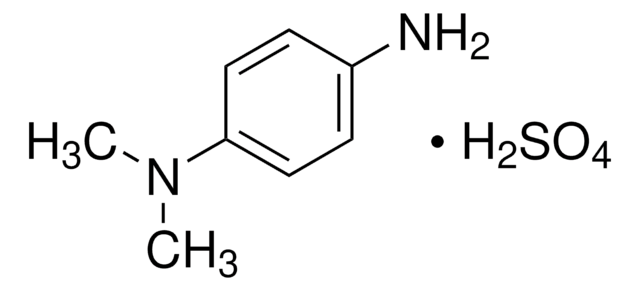
N,N-Diethyl-p-phenylenediamine sulfate salt
≥98.0% (T)
Synonym(s):
4-Amino-N,N-diethylaniline sulfate salt
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(C2H5)2NC6H4NH2 · H2SO4
CAS Number:
Molecular Weight:
262.33
Beilstein:
4219768
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
solid
mp
184-186 °C (lit.)
184-187 °C
SMILES string
OS(O)(=O)=O.CCN(CC)c1ccc(N)cc1
InChI
1S/C10H16N2.H2O4S/c1-3-12(4-2)10-7-5-9(11)6-8-10;1-5(2,3)4/h5-8H,3-4,11H2,1-2H3;(H2,1,2,3,4)
InChI key
AYLDJQABCMPYEN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N-Diethyl-p-phenylenediamine sulfate salt has been used to prepare N,N-diethyl-p-phenylenediamine (DPD) solution, which is commonly used as a chromogenic indicator for the determination of free and total chlorine by titrimetric, colorimetric and spectroscopic methods.{50
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An improved N, N?-diethyl-p-phenylenediamine (DPD) method for the determination of free chlorine based on multiple wavelength detection.
Moberg L & Karlberg B
Analytica Chimica Acta, 407(1-2), 127-133 (2000)
Improvements in the N, N-diethyl-p-phenylenediamine method for the determination of free and combined residual chlorine through the use of FIA.
Gordon G, et al.
Talanta, 38(2), 145-149 (1991)
Masoumeh Hasani et al.
Journal of hazardous materials, 157(1), 161-169 (2008-02-15)
A multicomponent analysis method based on principal component analysis-artificial neural network models (PC-ANN) is proposed for the determination of phenolic compounds. The method relies on the oxidative coupling of phenols (phenol, 2 chlorophenol, 3-chlorophenol and 4-chlorophenol) to N,N-diethyl-p-phenylenediamine in the
Jessica A Kershaw et al.
Analytical and bioanalytical chemistry, 379(4), 707-713 (2004-06-04)
The effect of Cu(II) on the determination of homocysteine via its electrochemically initiated reaction with N, N-diethyl-p-phenylenediamine is examined. The presence of copper inhibited the detection process for homocysteine owing to a complexation reaction occurring. This provided an indirect route
Z B Ogel et al.
Applied microbiology and biotechnology, 71(6), 853-862 (2006-01-04)
Scytalidium thermophilum produces an extracellular phenol oxidase on glucose-containing medium. Certain phenolic acids, specifically gallic acid and tannic acid, induce the expression of the enzyme. Production at 45 degrees C in batch cultures is growth-associated and is enhanced in the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service