A1153
Aprotinin
3-8 TIU/mg solid, lyophilized powder
Synonym(s):
BPTI, Bovine pancreatic trypsin inhibitor, Trasylol, Trypsin inhibitor (basic)
About This Item
Recommended Products
Product Name
Aprotinin from bovine lung, lyophilized powder, 3-8 TIU/mg solid
biological source
bovine lung
Quality Level
form
lyophilized powder
specific activity
3-8 TIU/mg solid
mol wt
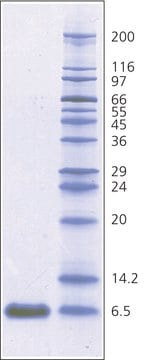
~6,500
solubility
H2O: ≥5 mg/mL
UniProt accession no.
storage temp.
2-8°C
SMILES string
S1SCC2NC(=O)CNC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C5NC(=O)C(NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C9N(CCC9)C(=O)CNC(=O)C(NC(=O)C(NC(=O)C%11N(CCC%11)C(=O
InChI
1S/C284H432N84O79S7/c1-21-144(9)222-271(439)337-174(68-46-105-309-282(300)301)239(407)340-187(120-160-77-85-164(374)86-78-160)251(419)341-185(116-156-55-29-24-30-56-156)250(418)342-188(121-161-79-87-165(375)88-80-161)252(420)346-191(123-208(291)378)246(41
InChI key
ZPNFWUPYTFPOJU-UHFFFAOYSA-N
Gene Information
cow ... PTI(404172)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a protease inhibitor in radioimmunoprecipitation assay buffer (RIPA) for the homogenization of cardiac microvascular endothelial cells (CMECs)(4) and mammary epithelial cells
- in angiogenesis assay for fibroblast
- in the proteomic stabilization of saliva supernatant
Biochem/physiol Actions
Unit Definition
Preparation Note
also commonly purchased with this product
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
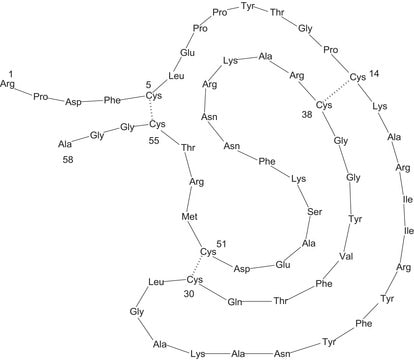
While aprotinin and bovine pancreatic trypsin inhibitor (BPTI) are the same protein sequence, the term aprotinin is typically used when describing the protein derived from bovine lung.
Elastase application index for understanding leukocyte elastase, a 29KDa serine endoprotease.
ReadyShield® phosphatase and protease inhibitor cocktail FAQ for sample protection in a variety of cell types and tissue extracts, including mammalian, plant, and microbial samples. Our ReadyShield® Protease Inhibitor Cocktail is a non-freezing solution that contains inhibitors with a broad specificity for serine, cysteine, acid proteases and aminopeptidases.
Analytical Enzyme Chymotrypsin: Chymotrypsin is produced in the acinar cells of the pancreas as the inactive precursor, chymotrypsinogen.
Protocols
Objective: To standardize a procedure for the enzymatic assay of Aprotinin.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service