G1163
O-Glycosidase from Streptococcus pneumoniae
recombinant, expressed in E. coli, buffered aqueous solution
Synonym(s):
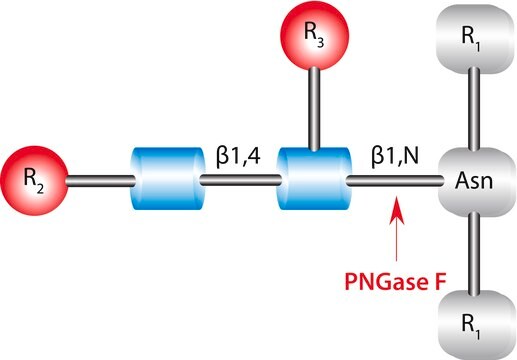
Endo-α-N-acetylgalactosaminidase, O-Glycanase
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
conjugate
(O-linked)
form
buffered aqueous solution
mol wt
180 kDa
concentration
≥800 units/mL
shipped in
wet ice
storage temp.
2-8°C
Biochem/physiol Actions
Packaging
Unit Definition
Physical form
Analysis Note
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Learn about O-linked glycan strategies, O-glycosidase actions, how to remove sialic acid residues, β-Elimination, and O-glycan modifications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service