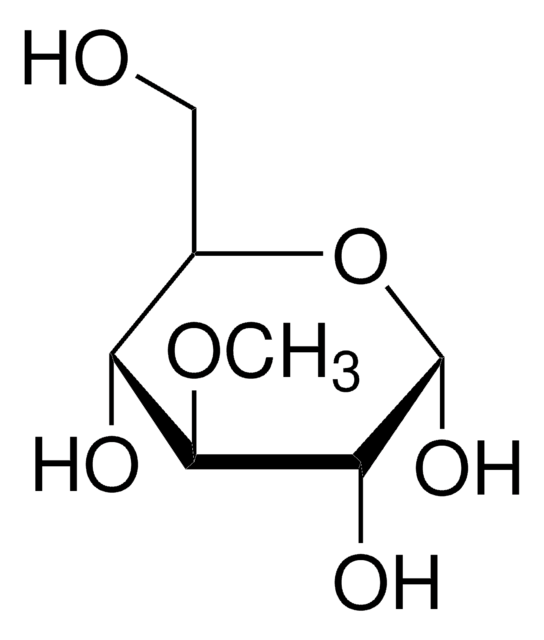
M0285
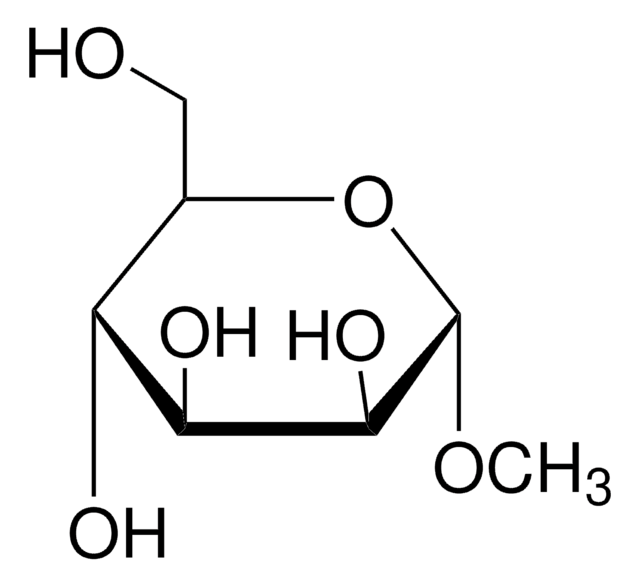
Methyl-β-D-galactopyranoside
Synonym(s):
Methyl β-D-galactoside
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H14O6
CAS Number:
Molecular Weight:
194.18
Beilstein:
81569
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic
Quality Level
Assay
≥98% (GC)
form
powder
technique(s)
gas chromatography (GC): suitable
color
white to faint yellow
mp
176-179 °C
solubility
water: 50 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O
InChI
1S/C7H14O6/c1-12-7-6(11)5(10)4(9)3(2-8)13-7/h3-11H,2H2,1H3/t3-,4+,5+,6-,7-/m1/s1
InChI key
HOVAGTYPODGVJG-VOQCIKJUSA-N
Looking for similar products? Visit Product Comparison Guide
General description
A β-D-galactopyranoside having a methyl substituent at the anomeric position.
Biochem/physiol Actions
Methyl galactoside is a hexose involved in the metabolism of 2-deoxygalactose.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J A Van Kuik et al.
European journal of biochemistry, 169(2), 399-411 (1987-12-01)
Hemocyanin from the freshwater snail Lymnaea stagnalis is a high-molecular-mass copper-containing glyco-protein which functions as oxygen carrier in the hemolymph. To release the carbohydrate chains, the protein was digested by pronase followed by hydrazinolysis and reduction. The oligosaccharide-alditols were purified
Satoru Takahashi et al.
Epilepsy research, 80(1), 18-22 (2008-05-06)
Glucose transporter 1 (GLUT1) deficiency syndrome is caused by a deficit in glucose transport to the brain during the pre- and postnatal periods. Here, we report two cases of GLUT1 deficiency syndrome diagnosed on the basis of clinical features, reduced
Alexander I Zinin et al.
Carbohydrate research, 337(7), 635-642 (2002-03-23)
1-O-Acetyl-beta-D-galactopyranose (AcGal), a new substrate for beta-galactosidase, was synthesized in a stereoselective manner by the trichloroacetimidate procedure. Kinetic parameters (K(M) and k(cat)) for the hydrolysis of 1-O-acetyl-beta-D-galactopyranose catalyzed by the beta-D-galactosidase from Penicillium sp. were compared with similar characteristics for
T J Kelley et al.
Bioscience reports, 19(5), 433-447 (2000-04-14)
The highly purified DNA Pol-alpha from rat prostate tumor (PA-3) and human neuroblastoma (IMR-32) cells appeared to be inhibited by Ricin (RCA-II), and Con-A. Loss of activity (40 to 60%) of a specific form of DNA polymerase from IMR-32 was
Feng Yang et al.
Carbohydrate research, 337(6), 485-491 (2002-03-14)
Oligosaccharide derivatives from sanqi, a Chinese herbal medicine derived from Panax notoginseng, methyl beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranoside, diosgenyl beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranoside, and methyl beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranosyl-(1-->4)-beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranoside, were synthesized under standard glycosylation conditions. An unexpected alpha-(1-->4) linkage was formed predominantly in the presence of neighboring participation group during
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service