M7397
L-(−)-Malic acid
suitable for cell culture, BioReagent, suitable for insect cell culture
Synonym(s):
(S)-(−)-2-Hydroxysuccinic acid, L-Hydroxybutanedioic acid
About This Item
Recommended Products
biological source
microbial
Quality Level
product line
BioReagent
Assay
≥95% (titration)
form
powder
technique(s)
cell culture | insect: suitable
cell culture | mammalian: suitable
mp
101-103 °C (lit.)
solubility
water: 100 mg/mL, clear to slightly hazy, colorless
SMILES string
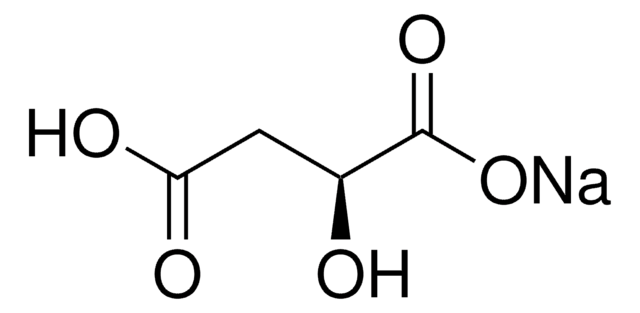
O[C@@H](CC(O)=O)C(O)=O
InChI
1S/C4H6O5/c5-2(4(8)9)1-3(6)7/h2,5H,1H2,(H,6,7)(H,8,9)/t2-/m0/s1
InChI key
BJEPYKJPYRNKOW-REOHCLBHSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to induce basal respiration in mitochondrial cell culture
- to measure the mitochondrial ETC (electron transport chain) activity
- as a component of mitochondrial substrate stock
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service