M8945
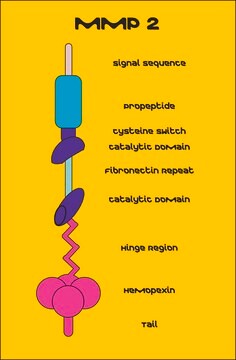
Matrix Metalloproteinase-9 human
recombinant, >90% (SDS-PAGE), buffered aqueous solution
Synonym(s):
Gelantinase-B, Gelatinase, 95 kDa, MMP-9
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Quality Level
Assay
>90% (SDS-PAGE)
form
buffered aqueous solution
mol wt
apparent mol wt ~93 kDa
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... MMP9(4318)
Biochem/physiol Actions
MMP-9 degrades the collagens that make up the extracellular matrix, which has a role in the control of angiogenesis.
Physical form
Solution in Tris-HCl, pH 7.5, Calcium Chloride, Sodium Chloride, and Brij-35
Analysis Note
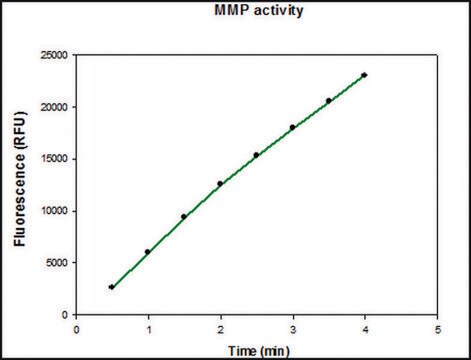
The biological activity is measured by its ability to cleave a fluorogenic peptide substrate.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carla Graça et al.
Foods (Basel, Switzerland), 9(10) (2020-10-07)
The influence of flour replacement by yogurt or curd-cheese additions (from 10% to 20%, w/w) on the glycemic response and bioactivity improvements of gluten-free bread was evaluated. Starch digestibility, measured by an in vitro digestion model, was applied to determine
Gelatinase B
Collier, I.E., and Goldberg, G.I. et al.
Handbook of Proteolytic Enzymes, 1205-1210 (1998)
S Chandler et al.
Neuroscience letters, 201(3), 223-226 (1995-12-15)
Matrix metalloproteinases (MMPs) are a group of enzymes responsible for the degradation of interstitial connective tissue and basement membrane. The coding sequences for five of the human MMPs, viz. interstitial collagenase, 72 kDa gelatinase, stromelysin-1, matrilysin and 92 kDa gelatinase
Wei-Li Hsu et al.
BMC veterinary research, 10, 202-202 (2014-08-28)
Neutrophil gelatinase-associated lipocalin (NGAL) is a useful biomarker for the early prediction of renal diseases. NGAL may exist as monomer, dimer and/or NGAL/MMP-9 complex forms in humans. In this study, the existence of various forms of NGAL in urine (uNGAL)
Tomoaki Fukui et al.
Biochemical and biophysical research communications, 460(3), 741-746 (2015-03-31)
Non-destructive detection of cartilage-degrading activities represents an advance in osteoarthritis (OA) research, with implications in studies of OA pathogenesis, progression, and intervention strategies. Matrix metalloproteinases (MMPs) are principal cartilage degrading enzymes that contribute to OA pathogenesis. MMPSense750 is an in-vivo
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service