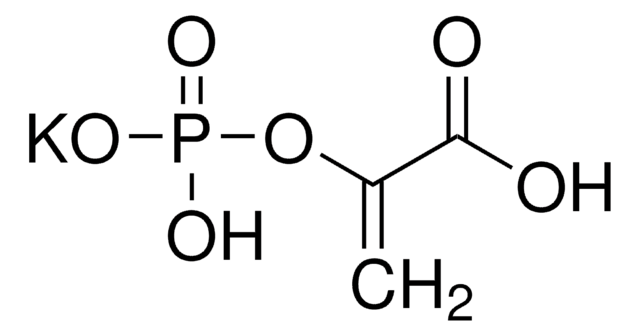
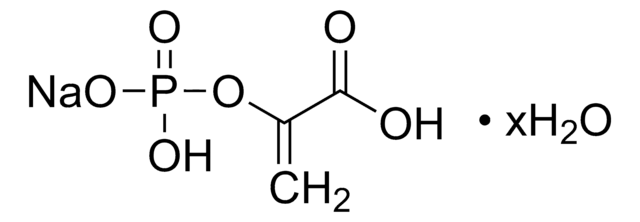
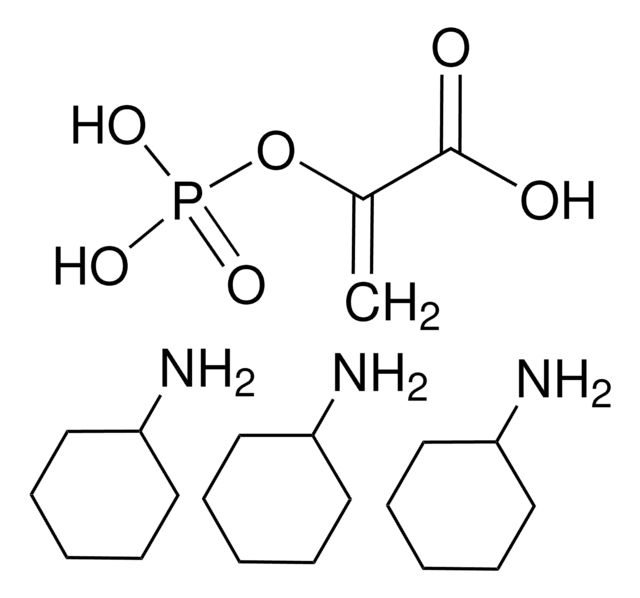
P0294
Pyruvate Kinase/Lactic Dehydrogenase enzymes from rabbit muscle
For the Determination of ADP, buffered aqueous glycerol solution
Synonym(s):
PK/LDH enzymes from rabbit muscle
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
form
buffered aqueous glycerol solution
mol wt
59 kDa
concentration
600-1,000 units/mL pyruvate kinase
900-1400 units/mL lactic dehydrogenase
storage temp.
−20°C
General description
Pyruvate Kinase from rabbit muscle is a metalloenzyme which catalyzes the conversion of phosphoenol pyruvate to pyruvate in the glycolysis pathway. It corresponds to a molecular weight of 59 kDa. It exists as a tetramer and undergoes conformational changes in the active site to accommodate substrate. Lactic dehydrogenase (LDH) catalyzes the lactate to pyruvate conversion in anaerobic glycolysis. It exists as tetramer and comprises of two subunits (H and M). The LDH of eukaryotes undergo active-site loop gating for their catalytic functionality.
Application
Pyruvate Kinase/Lactic Dehydrogenase enzymes from rabbit muscle has been used:
- for ATP generation in the active microtubule preparation
- in the enzyme linked ATPase assay of skeletal muscle heavy meromyosin (HMM)
- as a standard control for quantifying mesenchymal stem cells (MSCs) lactate dehydrogenase
Biochem/physiol Actions
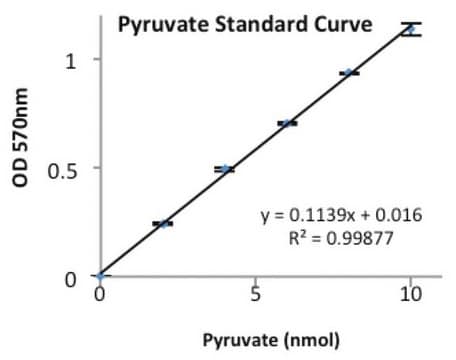
ADP Quantification Assay protocol for the use of PK/LDH in the determination of ADP. Solutions containing unkown concentrations of ADP can be substuted for reagent D in this protocol. Further dilutions of the ADP solution may be required
Lactate dehydrogenase from rabbit muscle can be inhibited by ascorbate. Aldolase and actin were shown to block this inhibitory effect.
Pyruvate kinase also catalyzes the phosphorylation of thiamine diphosphate (TDP) to thiamine triphosphate (TTP) which may find application in antiviral and tumor therapy.
Pyruvate kinase requires bivalent and monovalent cations such as Mg2+ and K+ respectively for activation to occur.
Unit Definition
Pyruvate kinase activity: One unit will convert 1.0 μmole of phospho(enol)pyruvate to pyruvate per min at pH 7.6 at 37 °C.
Lactic dehydrogenase activity: One unit will reduce 1.0 μmole of pyruvate to L-lactate per min at pH 7.5 at 37 °C.
Lactic dehydrogenase activity: One unit will reduce 1.0 μmole of pyruvate to L-lactate per min at pH 7.5 at 37 °C.
Physical form
Solution in 50% glycerol containing 10 mM HEPES, pH 7.0, 100 mM KCl and 0.1 mM EDTA
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thermal activation of `allosteric-like?large-scale motions in a eukaryotic Lactate Dehydrogenase
Katava M, et al.
Scientific Reports, 7, 41092-41092 (2017)
Chemical and enzymatic characterization of recombinant rabbit muscle pyruvate kinase
Boehme C, et al.
Biological Chemistry, 394(5), 695-701 (2013)
Jianming Zhang et al.
Nature, 463(7280), 501-506 (2010-01-15)
In an effort to find new pharmacological modalities to overcome resistance to ATP-binding-site inhibitors of Bcr-Abl, we recently reported the discovery of GNF-2, a selective allosteric Bcr-Abl inhibitor. Here, using solution NMR, X-ray crystallography, mutagenesis and hydrogen exchange mass spectrometry
Ligand-Induced Domain Movement in Pyruvate Kinase: Structure of the Enzyme from Rabbit Muscle with Mg2+, K+, and l-Phospholactate at 2.7 AA Resolution
Larsen TM, et al.
Archives of Biochemistry and Biophysics, 345(2), 199-206 (1997)
Characterization of rabbit lactate dehydrogenase-M and lactate dehydrogenase-H cDNAs. Control of lactate dehydrogenase expression in rabbit muscle.
Sass C, et al.
The Journal of Biological Chemistry, 264(7), 4076-4081 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service