R9156
Raltitrexed
≥98% (HPLC), solid
Synonym(s):
L-Glutamic acid, N-((5-(((1,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl)methylamino)-2-thienyl)carbonyl), ICI-D-1694, ZD-1694
About This Item
Recommended Products
Assay
≥98% (HPLC)
form
solid
color
off-white to light yellow
solubility
H2O: ≥10 mg/mL
DMSO: ≥40 mg/mL
originator
AstraZeneca
storage temp.
2-8°C
SMILES string
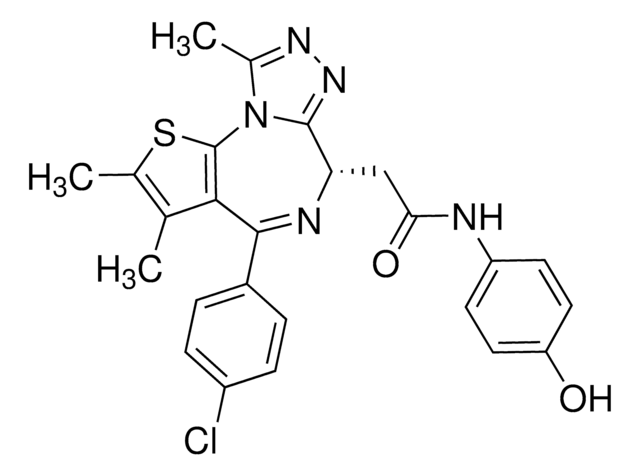
O=C1NC(C)=NC2=C1C=C(CN(C3=CC=C(C(N[C@H](CCC(O)=O)C(O)=O)=O)S3)C)C=C2
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Repr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Cancer research innovations address the complexity of the disease, providing advanced technologies for varied applications.
Biofiles reviews innovative technologies for cancer research, reflecting the complexity of the disease.
Cell cycle phases (G1, S, G2, M) regulate cell growth, DNA replication, and division in proliferating cells.
Apoptosis regulation involves multiple pathways and molecules for cellular homeostasis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service