All Photos(1)
About This Item
Empirical Formula (Hill Notation):
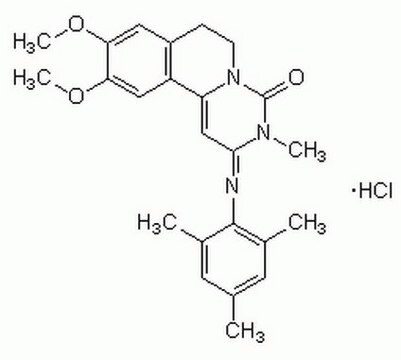
C24H27N3O3 · HCl
CAS Number:
Molecular Weight:
441.95
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98%
form
powder
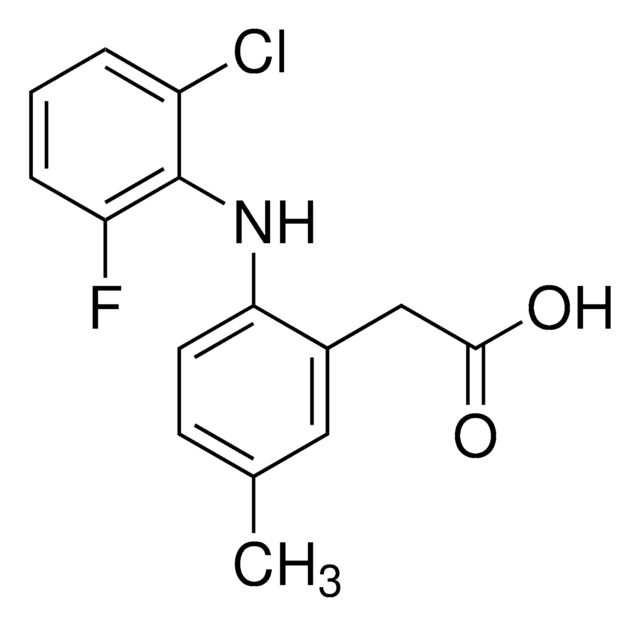
SMILES string
Cl[H].COc1cc2CCN3C(=O)N(C)C(\C=C3c2cc1OC)=N\c4c(C)cc(C)cc4C
InChI
1S/C24H27N3O3.ClH/c1-14-9-15(2)23(16(3)10-14)25-22-13-19-18-12-21(30-6)20(29-5)11-17(18)7-8-27(19)24(28)26(22)4;/h9-13H,7-8H2,1-6H3;1H/b25-22+;
InChI key
DTCZZBVPTHVXFA-OSMRDGEFSA-N
Gene Information
human ... PDE3A(5139) , PDE3B(5140)
Application
Trequinsin has been used as a PDE3 inhibitor in rat juxtaglomerular cells. This study reported that trequinsin can enhance cellular cAMP content, forskolin-induced cAMP synthesis, and renin release in cells.
Biochem/physiol Actions
Phosphodiesterase III inhibitor.
Trequinsin is a strong antihypertensive agent that has a hemodynamic profile similar to that of arteriolar dilators. Trequinsin can block platelet aggregation and also inhibit tissue factor expression in human endothelial cells,.
Features and Benefits
This compound is a featured product for Cyclic Nucleotide research. Click here to discover more featured Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Phosphodiesterases page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jorge E Torres-López et al.
European journal of pharmacology, 519(1-2), 75-79 (2005-08-23)
The local peripheral (subcutaneous) injection of phosphodiesterase 3 inhibitor trequinsin dose-dependently enhanced formalin-evoked flinching during late second phase of this test. Treatment with the nitric oxide synthase inhibitor N-L-nitro-arginine methyl ester or guanylyl cyclase inhibitor 1-H-[1,2,4,]oxadiazolo[4,3-a]quinoxalin-1-one significantly reversed trequinsin-induced pronociceptive
N E Leclerc et al.
Journal of cardiovascular pharmacology, 25 Suppl 2, S88-S91 (1995-01-01)
Exposure of endothelial cells (ECs) to thrombin or cytokines leads to major changes in their biochemical properties, which confer procoagulant activities. Stimulated ECs express the procoagulant glycoprotein tissue factor (TF). Although some TF is expressed on the apical surface of
R C Dunn et al.
FEBS letters, 238(2), 357-360 (1988-10-10)
Low-density lipoproteins activate isolated human platelets. The mechanism of this activation is unknown, but may involve increased phosphoinositide turnover. We have examined the effect of low-density lipoproteins on intracellular calcium concentrations in platelets loaded with the photoprotein aequorin. The lipoproteins
Shingo Tsukada et al.
Neuroscience letters, 318(1), 17-20 (2002-01-12)
To study the roles of nitric oxide (NO) in growth of nerve fibers, (+/-)-(E)-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexeneamine (NOR3), an NO-donor, was applied to cultured dorsal root ganglion (DRG) neurites from a micropipette. Ejection of a small volume of 1 mM NOR3 solution (not
Ulla G Friis et al.
Circulation research, 90(9), 996-1003 (2002-05-23)
We tested the hypothesis that cGMP stimulates renin release through inhibition of the cAMP-specific phosphodiesterase 3 (PDE3) in isolated rat juxtaglomerular (JG) cells. In addition, we assessed the involvement of PDE4 in JG-cell function. JG cells expressed PDE3A and PDE3B
Articles
Cyclic nucleotides like cAMP modulate cell function via PKA activation and ion channels.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service