151637
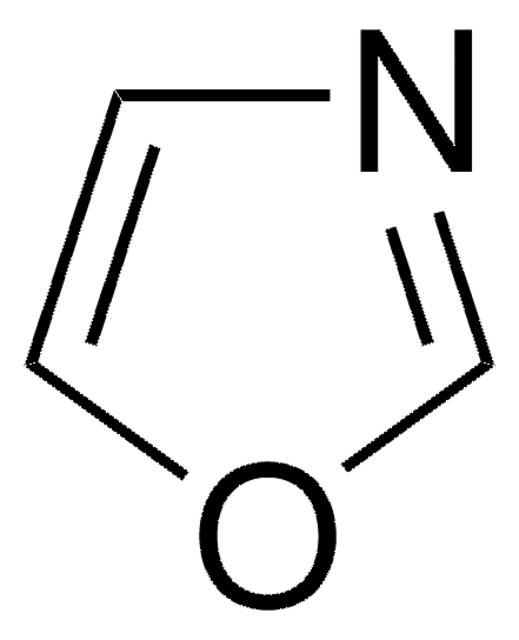
Isoxazole
99%
Synonym(s):
1,2-Oxazole, 1-Oxa-2-azacyclopentadiene, 2-Azafuran
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H3NO
CAS Number:
Molecular Weight:
69.06
Beilstein:
103773
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.4 (vs air)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.427 (lit.)
bp
93-95 °C (lit.)
density
1.078 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
c1cnoc1
InChI
1S/C3H3NO/c1-2-4-5-3-1/h1-3H
InChI key
CTAPFRYPJLPFDF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Isoxazole are described as inhibitors of acetylcholinesterase (AChE). Isoxazole ligands bind to and inhibit the Sxc- antiporter.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
53.6 °F - closed cup
Flash Point(C)
12 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Adam A Wallace et al.
The journal of physical chemistry. A, 125(1), 317-326 (2020-12-29)
Electron capture by the σ* LUMO of isoxazole triggers the dissociation of the O-N bond and the opening of the ring. Photodetachment of the resulting anion accesses a neutral structure, in which the O· and ·N bond fragments interact through
Margarita Gutiérrez et al.
The Journal of pharmacy and pharmacology, 65(12), 1796-1804 (2013-11-05)
Inhibition of acetylcholinesterase (AChE) is a common treatment for early stages of Alzheimer's disease. In this study, nine isoxazoles derivatives were tested for their in-vitro AChE activity. The molecular docking showed the interaction of the compounds with the active site.
Marina N Semenova et al.
ACS combinatorial science, 20(12), 700-721 (2018-11-20)
A series of both novel and reported combretastatin analogues, including diarylpyrazoles, -isoxazoles, -1,2,3-triazoles, and -pyrroles, were synthesized via improved protocols to evaluate their antimitotic antitubulin activity using in vivo sea urchin embryo assay and a panel of human cancer cells.
Ilaria Lampronti et al.
Oncology letters, 20(5), 151-151 (2020-09-17)
In order to develop potential anticancer agents stimulating apoptosis, novel 3,4-isoxazolediamide and 4,5,6,7-tetrahydro-isoxazolo-[4,5-c]-pyridine derivatives have been synthetized. The original structures of geldanamycin and radicicol, which are known natural heat shock protein (HSP) inhibitors, were deeply modified because both of them
Nikolai V Rostovskii et al.
The Journal of organic chemistry, 82(1), 256-268 (2016-12-16)
4-Aminopyrrole-3-carboxylates and pyrazine-2-carboxylates were synthesized from 5-alkoxyisoxazoles and 1-sulfonyl-1,2,3-triazoles by tuning the Rh(II) catalyst and the reaction conditions. The reaction in chloroform at 100 °C under Rh
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service